Chemistry:Canbisol
From HandWiki
Short description: Chemical compound
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
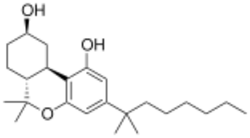
| Formula | C24H38O3 |
| Molar mass | 374.565 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Canbisol (Nabidrox), is a synthetic cannabinoid derivative that is the dimethylheptyl homologue of 9-nor-9β-hydroxyhexahydrocannabinol (HHC). It is a potent agonist at both the CB1 and CB2 receptors, with a binding affinity of 0.1 nM at CB1 and 0.2 nM at CB2.[1] It is mainly used in scientific research, in receptor binding studies to determine the structure and function of the cannabinoid receptors,[2][3][4] but has been made illegal in some countries due to its possible abuse potential as a cannabinomimetic drug.[5]
See also
References
- ↑ "Cannabinol derivatives: binding to cannabinoid receptors and inhibition of adenylylcyclase". Journal of Medicinal Chemistry 40 (20): 3228–33. September 1997. doi:10.1021/jm970126f. PMID 9379442.
- ↑ "Functional role of tryptophan residues in the fourth transmembrane domain of the CB(2) cannabinoid receptor". Journal of Neurochemistry 75 (6): 2485–91. December 2000. doi:10.1046/j.1471-4159.2000.0752485.x. PMID 11080201.
- ↑ "Functional role of serine residues of transmembrane dopamin VII in signal transduction of CB2 cannabinoid receptor". Journal of Veterinary Science 3 (3): 185–91. September 2002. doi:10.4142/jvs.2002.3.3.185. PMID 12514330.
- ↑ "Cysteine 2.59(89) in the second transmembrane domain of human CB2 receptor is accessible within the ligand binding crevice: evidence for possible CB2 deviation from a rhodopsin template". Molecular Pharmacology 68 (1): 69–83. July 2005. doi:10.1124/mol.104.007823. PMID 15840841.
- ↑ The Misuse of Drugs Act 1971 (Amendment) Order 2009
- ↑ "Enhancement of brain [3H]flunitrazepam binding and analgesic activity of synthetic cannabimimetics". European Journal of Pharmacology 109 (2): 201–12. February 1985. doi:10.1016/0014-2999(85)90421-2. PMID 2986995.
 |
