Chemistry:Testosterone phenylpropionate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Testolent, Sustanon 100, Sustanon 250, Omnadren 250 |
| Other names | TPP; Testosterone phenpropionate; Testosterone hydrocinnamate |
| Routes of administration | Intramuscular injection |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C28H36O3 |
| Molar mass | 420.593 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
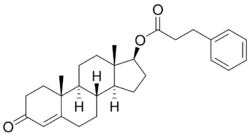
Testosterone phenylpropionate (BAN; TPP) (brand name Testolent), or testosterone phenpropionate, also known as testosterone hydrocinnamate, is a synthetic anabolic-androgenic steroid (AAS) and an androgen ester – specifically, the C17β phenylpropionate ester of testosterone – which was formerly marketed in Romania.[1][2][3][4] It was first synthesized in 1951 and was first described in the literature by 1953.[5] The medication was an ingredient of several isolated AAS commercial products, but was never widely used.[4] Testosterone phenylpropionate was also notably a component of Sustanon and Omnadren, as well as of Estandron Prolongatum, Lynandron Prolongatum, and Mixogen.[4][6] TPP was previously available in Great Britain.[7]
| Medication | Form | Major brand names | Duration |
|---|---|---|---|
| Testosterone | Aqueous suspension | Andronaq, Sterotate, Virosterone | 2–3 days |
| Testosterone propionate | Oil solution | Androteston, Perandren, Testoviron | 3–4 days |
| Testosterone phenylpropionate | Oil solution | Testolent | 8 days |
| Testosterone isobutyrate | Aqueous suspension | Agovirin Depot, Perandren M | 14 days |
| Mixed testosterone estersa | Oil solution | Triolandren | 10–20 days |
| Mixed testosterone estersb | Oil solution | Testosid Depot | 14–20 days |
| Testosterone enanthate | Oil solution | Delatestryl | 14–20 days |
| Testosterone cypionate | Oil solution | Depovirin | 14–20 days |
| Mixed testosterone estersc | Oil solution | Sustanon 250 | 28 days |
| Testosterone undecanoate | Oil solution | Aveed, Nebido | 100 days |
| Testosterone buciclated | Aqueous suspension | 20 Aet-1, CDB-1781e | 90–120 days |
| Nandrolone phenylpropionate | Oil solution | Durabolin | 10 days |
| Nandrolone decanoate | Oil solution | Deca Durabolin | 21 days |
| Methandriol | Aqueous suspension | Notandron, Protandren | 8 days |
| Methandriol bisenanthoyl acetate | Oil solution | Notandron Depot | 16 days |
| Metenolone acetate | Oil solution | Primobolan | 3 days |
| Metenolone enanthate | Oil solution | Primobolan Depot | 14 days |
| Note: All are via i.m. injection. Footnotes: a = TP, TV, and TUe. b = TP and TKL. c = TP, TPP, TiCa, and TD. d = Studied but never marketed. e = Developmental code names. Sources: See template. | |||
See also
References
- ↑ The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 641–642. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA641.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA976.
- ↑ Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA270.
- ↑ 4.0 4.1 4.2 Anabolics. Molecular Nutrition Llc. 2011. pp. 583, 697. ISBN 978-0-9828280-1-4. https://books.google.com/books?id=afKLA-6wW0oC&pg=PT697.
- ↑ "Testosterone phenyl propionate (TPP): biological trials with a new androgen". Br J Pharmacol Chemother 8 (3): 271–7. September 1953. doi:10.1111/j.1476-5381.1953.tb00793.x. PMID 13093945.
- ↑ "Clinical Use and Abuse of Androgens and Antiandrogens". Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. 2001. pp. 1185–. ISBN 978-0-7817-1750-2. https://books.google.com/books?id=FVfzRvaucq8C&pg=PA1185.
- ↑ "Endocrine Treatment of Gynaecological Disorders". Modern Trends in Endocrinology. 1. London: Butterworth & Co.. 1958. pp. 231–244. https://books.google.com/books?id=bDNBAAAAYAAJ.
{{Navbox
| name = Androgens and antiandrogens | title = Androgens and antiandrogens | state = collapsed | listclass = hlist | groupstyle = text-align:center;
| group1 = Androgens
(incl. AAS)
| list1 =
| group2 = Antiandrogens | list2 = {{Navbox|child | groupstyle = text-align:center; | groupwidth = 9em;
| group1 = AR antagonists | list1 =
- Steroidal: Abiraterone acetate
- Canrenone
- Chlormadinone acetate
- Cyproterone acetate
- Delmadinone acetate
- Dienogest
- Drospirenone
- Medrogestone
- Megestrol acetate
- Nomegestrol acetate
- Osaterone acetate
- Oxendolone
- Potassium canrenoate
- Spironolactone
- Nonsteroidal: Apalutamide
- Bicalutamide
- Cimetidine
- Darolutamide
- Enzalutamide
- Flutamide
- Ketoconazole
- Nilutamide
- Seviteronel†
- Topilutamide (fluridil)
| group2 = Steroidogenesis| list2 =
inhibitors
| 5α-Reductase | |
|---|---|
| Others |
| group3 = Antigonadotropins | list3 =
- D2 receptor antagonists (prolactin releasers) (e.g., domperidone, metoclopramide, risperidone, haloperidol, chlorpromazine, sulpiride)
- Estrogens (e.g., bifluranol, [[diethylstilbestrol, estradiol, estradiol esters, ethinylestradiol, ethinylestradiol sulfonate, paroxypropione)
- GnRH agonists (e.g., leuprorelin)
- GnRH antagonists (e.g., cetrorelix)
- Progestogens (incl., chlormadinone acetate, [[cyproterone acetate, hydroxyprogesterone caproate, gestonorone caproate, [[Chemistry:Medroxyprogesterone medroxyprogesterone acetate, Chemistry:Megestrol acetate|megestrol acetate]])
| group4 = Others | list4 =
- Androstenedione immunogens: Androvax (androstenedione albumin)
- Ovandrotone albumin (Fecundin)
}}
| liststyle = background:#DDDDFF;| list3 =
- #WHO-EM
- ‡Withdrawn from market
- Clinical trials:
- †Phase III
- §Never to phase III
- See also
- Androgen receptor modulators
- Estrogens and antiestrogens
- Progestogens and antiprogestogens
- List of androgens/anabolic steroids
}}
 |
