Chemistry:O-1812
From HandWiki
Short description: Chemical compound
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
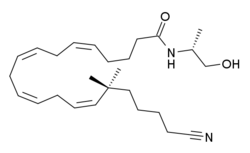
| Formula | C26H42N2O2 |
| Molar mass | 414.634 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
O-1812 is an eicosanoid derivative related to anandamide that acts as a potent and highly selective agonist for the cannabinoid receptor CB1, with a Ki of 3.4 nM at CB1 and 3870 nM at CB2.[1] Unlike most related compounds, O-1812 is metabolically stable against rapid breakdown by enzymes, and produces a cannabinoid-like discriminative effect in rats, which is similar but not identical to that produced by cannabinoid drugs of other chemical classes.[2][3][4][5]
See also
References
- ↑ "Highly selective CB1 cannabinoid receptor ligands and novel CB1/VR1 vanilloid receptor "hybrid" ligands". Biochemical and Biophysical Research Communications 281 (2): 444–51. February 2001. doi:10.1006/bbrc.2001.4354. PMID 11181068.
- ↑ "Differential effects of Δ9-tetrahydrocannabinol and methanandamide in CB1 knockout and wild-type mice". The Journal of Pharmacology and Experimental Therapeutics 309 (1): 86–91. April 2004. doi:10.1124/jpet.103.055376. PMID 14718593.
- ↑ "A comparison of the discriminative stimulus effects of Δ9-tetrahydrocannabinol and O-1812, a potent and metabolically stable anandamide analog, in rats". Experimental and Clinical Psychopharmacology 12 (3): 173–9. August 2004. doi:10.1037/1064-1297.12.3.173. PMID 15301634.
- ↑ "Task specificity of cross-tolerance between Δ9-tetrahydrocannabinol and anandamide analogs in mice". European Journal of Pharmacology 510 (1–2): 59–68. March 2005. doi:10.1016/j.ejphar.2005.01.006. PMID 15740725.
- ↑ "Sensitivity to Δ9-tetrahydrocannabinol is selectively enhanced in β-arrestin2 -/- mice". Behavioural Pharmacology 19 (4): 298–307. July 2008. doi:10.1097/FBP.0b013e328308f1e6. PMID 18622177.
 |

