Chemistry:Delta-3-Tetrahydrocannabinol
From HandWiki
Short description: Chemical compound
 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C21H30O2 |
| Molar mass | 314.469 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
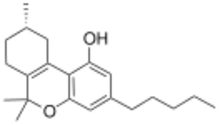
Delta-3-Tetrahydrocannabinol (Delta-3-THC, Δ3-THC, Δ6a(10a)-THC, EA-1477) is a synthetic isomer of tetrahydrocannabinol, developed during the original research in the 1940s to develop synthetic routes to the natural products Δ8-THC and Δ9-THC found in the cannabis plant.[1] While the normal trans configuration of THC is in this case flattened by the double bond, it still has two enantiomers as the 9-methyl group can exist in an (R) or (S) conformation. The (S) enantiomer has similar effects to Δ9-THC though with several times lower potency, while the (R) enantiomer is many times less active or inactive, depending on the assay used.[2][3][4] It has been identified as a component of vaping liquid products.[5]
See also
- 7,8-Dihydrocannabinol
- Cannabitriol
- Delta-4-Tetrahydrocannabinol
- Delta-7-Tetrahydrocannabinol
- Delta-10-Tetrahydrocannabinol
- Hexahydrocannabinol
- JWH-138
- Parahexyl
References
- ↑ Adams R, "Marihuana active compounds.", US patent 2419935, issued 1947
- ↑ "Cannabinoids. 1. 1-Amino- and 1-mercapto-7,8,9,10-tetrahydro-6H-dibenzo [b,d]pyrans". Journal of Medicinal Chemistry 20 (1): 17–24. January 1977. doi:10.1021/jm00211a004. PMID 833820.
- ↑ "Use of a potential rabbit model for structure--behavioral activity studies of cannabinoids". Journal of Medicinal Chemistry 25 (5): 596–9. May 1982. doi:10.1021/jm00347a021. PMID 7086846.
- ↑ "Base-catalysed double-bond isomerizations of cannabinoids: structural and stereochemical aspects.". Journal of the Chemical Society, Perkin Transactions 1: 2881–6. 1984. doi:10.1039/P19840002881.
- ↑ "EVALI Vaping Liquids Part 1: GC-MS Cannabinoids Profiles and Identification of Unnatural THC Isomers". Frontiers in Chemistry 9: 746479. 2021. doi:10.3389/fchem.2021.746479. PMID 34631667.

