Chemistry:KM-233
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C25H30O2 |
| Molar mass | 362.513 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
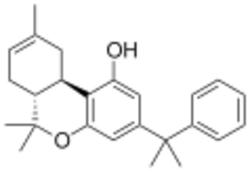
KM-233 is a synthetic cannabinoid drug which is a structural analog of Δ8-tetrahydrocannabinol (THC), the less active but more stable isomer of the active component of Cannabis. KM-233 differs from Δ8-THC by the pentyl side chain being replaced by a 1,1-dimethylbenzyl group. It has high binding affinity in vitro for both the CB1 and CB2 receptors, with a CB2 affinity of 0.91 nM and 13-fold selectivity over the CB1 receptor.[1] In animal studies, it has been found to be a potential treatment for glioma, a form of brain tumor.[2] Many related analogues are known where the 1,1-dimethylbenzyl group is substituted or replaced by other groups, with a fairly well established structure-activity relationship.[3][4][5][6][7]
See also
References
- ↑ "Synthesis and testing of novel phenyl substituted side-chain analogues of classical cannabinoids". Bioorganic & Medicinal Chemistry Letters 13 (20): 3487–90. October 2003. doi:10.1016/s0960-894x(03)00729-7. PMID 14505654.
- ↑ "Safety and efficacy of a novel cannabinoid chemotherapeutic, KM-233, for the treatment of high-grade glioma". Journal of Neuro-Oncology 77 (2): 143–52. April 2006. doi:10.1007/s11060-005-9031-y. PMID 16314952.
- ↑ "Synthesis and testing of novel classical cannabinoids: exploring the side chain ligand binding pocket of the CB1 and CB2 receptors". Bioorganic & Medicinal Chemistry 11 (14): 3121–32. July 2003. doi:10.1016/s0968-0896(03)00238-4. PMID 12818675.
- ↑ "The application of 3D-QSAR studies for novel cannabinoid ligands substituted at the C1' position of the alkyl side chain on the structural requirements for binding to cannabinoid receptors CB1 and CB2". Journal of Medicinal Chemistry 50 (12): 2875–85. June 2007. doi:10.1021/jm0610705. PMID 17521177.
- ↑ "Exploring the substituent effects on a novel series of C1'-dimethyl-aryl Delta8-tetrahydrocannabinol analogs". Bioorganic & Medicinal Chemistry 16 (13): 6489–500. July 2008. doi:10.1016/j.bmc.2008.05.034. PMID 18524604.
- ↑ "Quantitative structure-activity relationship (QSAR) for a series of novel cannabinoid derivatives using descriptors derived from semi-empirical quantum-chemical calculations". Bioorganic & Medicinal Chemistry 17 (6): 2598–606. March 2009. doi:10.1016/j.bmc.2008.11.059. PMID 19250829.
- ↑ "Three-dimensional quantitative structure-selectivity relationships analysis guided rational design of a highly selective ligand for the cannabinoid receptor 2". European Journal of Medicinal Chemistry 46 (2): 547–55. February 2011. doi:10.1016/j.ejmech.2010.11.034. PMID 21183257.
 |

