Chemistry:3'-Hydroxy-THC
From HandWiki
Short description: Chemical compound
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C21H30O3 |
| Molar mass | 330.468 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
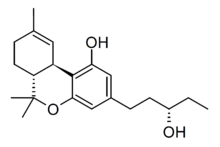
3'-Hydroxy-THC (3'-OH-Δ9-THC) is a minor active metabolite of THC, the main psychoactive component of cannabis. It is one of a number of metabolites of THC hydroxylated on the pentyl side chain, but while the other side-chain hydroxyl isomers are much weaker or inactive, the S enantiomer of 3'-OH-THC is several times more potent than THC itself, and while it is produced in smaller amounts than other active metabolites such as 11-Hydroxy-THC and 8,11-Dihydroxy-THC, it is thought to contribute to the overall pharmacological profile of cannabis.[1][2][3][4][5]
See also
References
- ↑ "Metabolism of delta1-tetrahydrocannabinol by the isolated perfused dog lung. Comparison with in vitro liver metabolism". The Journal of Pharmacy and Pharmacology 27 (11): 842–8. November 1975. doi:10.1111/j.2042-7158.1975.tb10227.x. PMID 1493.
- ↑ "Cannabinoids: Metabolites hydroxylated in the pentyl side chain.". Marihuana. Berlin, Heidelberg: Springer. 1976. pp. 141–157. doi:10.1007/978-3-642-51624-5_12. ISBN 978-3-642-51626-9.
- ↑ "3'-Hydroxy- and (+/-)-3',11-dihydroxy-delta 9-tetrahydrocannabinol: biologically active metabolites of delta 9-tetrahydrocannabinol". Journal of Medicinal Chemistry 25 (12): 1447–50. December 1982. doi:10.1021/jm00354a011. PMID 6296389.
- ↑ "Pharmacological potency of R- and S-3'-hydroxy-delta 9-tetrahydrocannabinol: additional structural requirement for cannabinoid activity". Pharmacology, Biochemistry, and Behavior 21 (1): 61–5. July 1984. doi:10.1016/0091-3057(84)90131-x. PMID 6087379.
- ↑ "Human cannabinoid pharmacokinetics". Chemistry & Biodiversity 4 (8): 1770–804. August 2007. doi:10.1002/cbdv.200790152. PMID 17712819.
 |

