Chemistry:Cannabicitran
From HandWiki
Short description: Chemical compound
 | |
 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C21H30O2 |
| Molar mass | 314.469 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
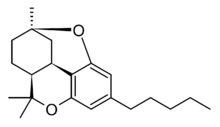
Cannabicitran (CBTC) is a phytocannabinoid first isolated in 1974 as a trace component of Cannabis sativa,[1][2][3][4] Structurally related compounds can be found in some other plants.[5][6] It is not psychoactive, but was found to reduce intraocular pressure in tests on rabbits,[7] which may reflect agonist activity at the NAGly receptor (formally GPR18) that is known to be a target of many structurally related cannabinoids.[8]
See also
- 9-OH-HHC
- Cannabichromene
- Cannabicyclol
- Cannabidiol dimethyl ether
- Cannabielsoin
- Cannabigerol
- Cannabimovone
- Cannabitriol
- Isotetrahydrocannabinol
References
- ↑ "Cannabicitran: a new naturally occurring tetracyclic diether from Lebanese Cannabis sativa.". Phytochemistry 13 (3): 619–21. March 1974. doi:10.1016/S0031-9422(00)91362-1.
- ↑ "Flavonoid glycosides and cannabinoids from the pollen of Cannabis sativa L". Phytochemical Analysis 16 (1): 45–8. 2005. doi:10.1002/pca.809. PMID 15688956.
- ↑ "Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis". Molecules 26 (9): 2774. May 2021. doi:10.3390/molecules26092774. PMID 34066753.
- ↑ "Phytocannabinoids: a unified critical inventory". Natural Product Reports 33 (12): 1357–1392. November 2016. doi:10.1039/c6np00074f. PMID 27722705.
- ↑ "Efficient Synthesis of Polycycles by Electrocyclizations of Substituted Trihydroxybenzenes: Synthesis of Rubranine and Deoxybruceol.". Synlett 2007 (14): 2232–2236. 2007. doi:10.1055/s-2007-985562.
- ↑ "New cannabinoid-like chromane and chromene derivatives from Rhododendron anthopogonoides". Chemical & Pharmaceutical Bulletin 59 (11): 1409–12. 2011. doi:10.1248/cpb.59.1409. PMID 22041081.
- ↑ "Cannabinoids in glaucoma II: the effect of different cannabinoids on intraocular pressure of the rabbit". Current Eye Research 3 (6): 841–50. June 1984. doi:10.3109/02713688409000797. PMID 6329602.
- ↑ "A GPR18-based signalling system regulates IOP in murine eye". British Journal of Pharmacology 169 (4): 834–43. June 2013. doi:10.1111/bph.12136. PMID 23461720.
 |

