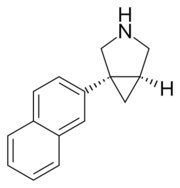
Chemistry:Centanafadine
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C15H15N |
| Molar mass | 209.292 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| Site | IC50 (nM) | Action | Ref |
|---|---|---|---|
| SERT | 83 nM | Blocker | [1] |
| NET | 6 nM | Blocker | [1] |
| DAT | 38 nM | Blocker | [1] |
Centanafadine (INN) (former developmental code name EB-1020) is a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI) that began its development with Euthymics Bioscience after they acquired DOV Pharmaceutical. It was developed as a treatment for attention-deficit hyperactivity disorder (ADHD) and inhibits the reuptake of norepinephrine, dopamine, and serotonin with a ratio of 1:6:14, respectively.[1][2][3][4] In 2011, Euthymics Bioscience spun off its development of centanafadine to a new company called Neurovance.[5][6] In March 2017, Otsuka Pharmaceutical acquired Neurovance and the rights to centanafadine.[7] As of January 2018, Otsuka's pipeline indicates it is in Phase II and III clinical trials for a number of different applications to medical conditions.[8][9][10]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Neurovance's EB-1020 SR for Adult ADHD Shows Stimulant-Like Efficacy and Good Tolerability in Phase 2a Trial". Neurovance. http://www.neurovance.com/wp-content/uploads/2014/05/Press_Release_Neurovance_EB-1020_Data_050714.pdf.
- ↑ "3-Neurotransmitters, 1-Molecule: Optimized Ratios". Neurovance. http://www.neurovance.com/science/.
- ↑ "EB-1020, a Non-Stimulant Norepinephrine and Dopamine - Preferring Reuptake Inhibitor for the Treatment of Adult ADHD". Neurovance. http://euthymics.com/wp-content/uploads/2012/10/White-Paper-EB1020-092612.pdf.
- ↑ "Pharmacological characterization of the norepinephrine and dopamine reuptake inhibitor EB-1020: implications for treatment of attention-deficit hyperactivity disorder". Synapse 66 (6): 522–32. June 2012. doi:10.1002/syn.21538. PMID 22298359.
- ↑ "Euthymics". http://euthymics.com/.
- ↑ "EUTHYMICS BIOSCIENCE, INC. PRESENTS DATA THAT SUPPORT ADVANCING EB-1020 INTO CLINICAL TRIALS FOR ADULT ADHD". Neurovance. December 7, 2011. http://www.neurovance.com/wp-content/uploads/2014/04/Neurovance_120711.pdf.
- ↑ "Otsuka Pharmaceutical to Acquire Neurovance, Inc.". Otsuka. https://www.otsuka-us.com/discover/articles-1000.
- ↑ "Otsuka U.S. Research & Development Programs". Otsuka. https://www.otsuka-us.com/media/images/AR-5-11-2017-OTS-US-Pipeline-Graphic-v02_959.jpg.[yes|permanent dead link|dead link}}]
- ↑ Otsuka Pharmaceutical Development & Commercialization, Inc. (2021-09-17). A Phase 3, Randomized, Double-blind, Multicenter, Placebo-controlled, Parallel-group Trial Evaluating the Efficacy, Safety, and Tolerability of Centanafadine Sustained-release Tablets in Adults With Attention-deficit/Hyperactivity Disorder. https://clinicaltrials.gov/ct2/show/results/NCT03605680.
- ↑ Gunduz-Bruce, Handan (2018-09-26). "SAGE-217 in major depressive disorder: a multicenter, randomized, double-blind, Phase 2 placebo-controlled trial". doi:10.26226/morressier.5b68175eb56e9b005965c44b. http://dx.doi.org/10.26226/morressier.5b68175eb56e9b005965c44b.
External links
 |

