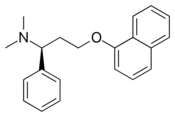
Chemistry:Dapoxetine
 | |
| Clinical data | |
|---|---|
| Trade names | EJ-30, Priligy, others (see below) |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral (tablets) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 15–76% (mean 42%), Tmax = 1–1.3 hours |
| Protein binding | >99% |
| Metabolism | Liver (CYP2D6, CYP3A4), kidney (FMO1) |
| Metabolites | Dapoxetine-N-oxide, desmethyldapoxetine, didesmethyldapoxetine |
| Elimination half-life | 1.5–1.6 h |
| Excretion | Kidneys[1] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H23NO |
| Molar mass | 305.421 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Dapoxetine, marketed as Priligy, among others, is a medication used for the treatment of premature ejaculation (PE) in men 18–64 years old.[2][3][4] Dapoxetine works by inhibiting the serotonin transporter, increasing serotonin's action at the postsynaptic cleft, and as a consequence promoting ejaculatory delay.[5] As a member of the selective serotonin reuptake inhibitor (SSRI) family, dapoxetine was initially created as an antidepressant. However, unlike other SSRIs, dapoxetine is absorbed and eliminated rapidly in the body. Its fast-acting property makes it suitable for the treatment of PE, but not as an antidepressant.[6]
Originally created by Eli Lilly pharmaceutical company, dapoxetine was sold to Johnson & Johnson in 2003 and submitted as a New Drug Application to the Food and Drug Administration (FDA) for the treatment of PE in 2004.[7] Dapoxetine is sold in several European and Asian countries, and in Mexico. In the US, dapoxetine has been in phase III development. In May 2012, US-based Furiex Pharmaceuticals reached an agreement with ALZA Corp and Janssen Pharmaceutica to market dapoxetine in the USA, Japan, and Canada, while selling the rights to market the drug in Europe, most of Asia, Africa, Latin America, and the Middle East to Menarini.[8]
Medical uses
Premature ejaculation
Randomized, double-blind, placebo-controlled trials have confirmed the efficacy of dapoxetine for the treatment of PE.[9] Different dosages have different impacts on different types of PE. Dapoxetine 60 mg significantly improves the mean intravaginal ejaculation latency time (IELT) compared to that of dapoxetine 30 mg in men with lifelong PE, but no difference is seen in men with acquired PE.[10] Dapoxetine, given 1–3 hours before sexual episode, prolongs IELT and increases the sense of control and sexual satisfaction in men of 18 to 64 years of age with PE. Since PE is associated with personal distress and interrelationship difficulty, dapoxetine provides help for men with PE to overcome this condition.[11] With no drug approved specifically for treatment for PE in the US and some other countries, other SSRIs such as fluoxetine, paroxetine, sertraline, fluvoxamine, and citalopram have been used off-label to treat PE. Waldinger's meta-analysis shows that the use of these conventional antidepressants increases IELT two- to nine-fold above baseline, compared to three- to eight-fold when dapoxetine is used.[10] However, these SSRIs may need to be taken daily to achieve meaningful efficacy, and their comparatively longer half-lives increase the risk of drug accumulation and a corresponding increase of adverse effects such as reduced libido.[12] Dapoxetine, though, is generally categorized as a fast-acting SSRI. It is more rapidly absorbed and mostly eliminated from the body within a few hours. These pharmacokinetics are more favorable in that they might minimize drug accumulation in the body, habituation, and side effects.[6]
Depression and anxiety
Although it was initially thought of as unsuccessful in its intended use as an antidepressant, dapoxetine has been more recently investigated as a possible aid to one approach to depression treatment focused on stress reduction, based on an animal model of depression.[13][14]
Contraindications
Dapoxetine should not be used in men with moderate to severe hepatic impairment and in those receiving CYP3A4 inhibitors such as ketoconazole, ritonavir, and telithromycin. Dapoxetine also cannot be used in patients with heart failure, permanent pacemaker, or other significant ischemic heart disease. Caution is advised in men receiving thioridazine, monoamine oxidase inhibitors, SSRIs, serotonin-norepinephrine reuptake inhibitors, or tricyclic antidepressants. If a patient stops taking one of these drugs, he should wait for 14 days before taking dapoxetine. If a patient stops taking dapoxetine, he should wait for 7 days before receiving these drugs.[5]
Adverse effects
The most common effects when taking dapoxetine are nausea, dizziness, dry mouth, headache, diarrhea, and insomnia.[15][16] Discontinuation rates due to adverse effects and costs are very high. A recent study in Asia showed that cumulative discontinuation rates within one year are as high as 87%.[17] Unlike other SSRIs used to treat depression, which have been associated with high incidences of sexual dysfunction,[18] dapoxetine is associated with low rates of sexual dysfunction. Taken as needed, dapoxetine has very mild adverse effects of decreased libido (<1%) and erectile dysfunction (<4%).[6]
Overdose
No case of drug overdose has been reported during clinical trials.[19]
Interactions
With phosphodiesterase inhibitors (PDE5 inhibitors)
Many men who have PE also suffer from erectile dysfunction (ED). Treatment for these patients should consider the drug–drug interaction between dapoxetine and PDE5 inhibitors such as tadalafil (Cialis) or sildenafil (Viagra). In Dresser study (2006), plasma concentration of 24 subjects was obtained. Half of the sample pool were treated with dapoxetine 60 mg plus tadalafil 20 mg; the other half were treated with dapoxetine 60 mg plus sildenafil 100 mg. These plasma samples were then analyzed using liquid chromatography-tandem mass spectrometry. The results showed that dapoxetine does not alter the pharmacokinetic of tadalafil or sildenafil.[2]
With ethanol
Ethanol does not affect the pharmacokinetics of dapoxetine when taking concurrently.[20]
Mechanism of action
The mechanism through which dapoxetine affects premature ejaculation is still unclear, but dapoxetine is presumed to work by inhibiting serotonin transporter and subsequently increasing serotonin's action at pre- and postsynaptic receptors.[21] Human ejaculation is regulated by various areas in the central nervous system (CNS).[22] The ejaculatory pathway originates from spinal reflex at the thoracolumbar and lumbosacral level of spinal cord activated by stimuli from male genitalia. These signals are passed on to the brain stem, which then is influenced by a number of nuclei in the brain such as medial preoptic and paraventricular nuclei.[23] Clement's study performed on anaesthetized male rats showed that acute administration of dapoxetine inhibits ejaculatory expulsion reflex at supraspinal level by modulating activity of lateral paragigantocellular nucleus (LPGi) neurons. These effects cause an increase in pudendal motoneuron reflex discharge (PMRD) latency, though whether dapoxetine acts directly on LPGi or on the descending pathway in which LPGi located is unclear.[24]
Pharmacokinetics
- Absorption
Dapoxetine is a white, powdery, water-insoluble substance. Taken one to three hours before sexual activity, it is rapidly absorbed in the body. Its maximum plasma concentration (Cmax) is reached one to two hours after oral administration. The Cmax and AUC (area under the plasma vs. time curve) is dose dependent. The Cmax and Tm (time needed to obtain the maximum plasma concentration) after single doses of dapoxetine 30 mg and 60 mg are 297 and 498 ng/ml at 1.01 and 1.27 hours, respectively. A high-fat meal does reduce the Cmax slightly, but it is insignificant. In fact, food does not alter dapoxetine pharmacokinetics. It can be taken with or without food.[25]
- Distribution
Dapoxetine is absorbed and distributed rapidly in the body. Greater than 99% of dapoxetine is bound to the plasma protein. The mean steady-state volume is 162 L. Its initial half-life is 1.31 hours (30 mg dose) and 1.42 hours (60 mg dose), and its terminal half life is 18.7 hours (30 mg dose) and 21.9 hours (60 mg dose).[26]
- Metabolism
Dapoxetine is metabolized extensively in the liver and kidney by multiple enzymes such as CYP2D6, CYP3A4, and flavin monooxygenase 1. The major product at the end of the metabolic pathway is circulating dapoxetine N-oxide, which is a weak SSRI and contributes no clinical effect. The other products presented less than 3% in the plasma are desmethyldapoxetine and didesmethydapoxetine. Desmethyldapoxetine is roughly equipotent to dapoxetine.[27]
- Excretion
The metabolites of dapoxetine are eliminated rapidly in the urine with a terminal half-life of 18.7 and 21.9 hours for a single dose of 30 mg and 60 mg, respectively.[20]
Safety and tolerability
- Cardiovascular safety
The cardiovascular safety profile of dapoxetine has been studied extensively during the drug development. Phase I trials showed that dapoxetine had neither clinically significant electrocardiographic effects nor delayed repolarization effects, with dosing up to four-fold greater than the maximum recommended dosage, which is 60 mg. Phase III studies in men with PE showed a safety and well tolerated profile of dapoxetine with dosing of 30 and 60 mg. No cardiovascular adverse had been found.[28]
- Neurocognitive safety
Studies of SSRIs in patients with major psychiatric disorders prove that SSRIs are potentially associated with certain neurocognitive adverse effects such as anxiety, akathisia, hypomania, changes in mood, or suicidal thought.[29][30] No study on the effects of SSRIs in men with PE has been done. McMahon's study in 2012 showed that dapoxetine has no effect on mood and is not associated with anxiety or suicidality.[31]
- Withdrawal syndrome
The incidence of antidepressant discontinuation syndrome symptoms in men using dapoxetine to treat PE has been described by reviewers as low or no different from the incidence of such symptoms in men withdrawn from placebo treatment.[32][33] The lack of chronic serotonergic stimulation with on-demand dapoxetine minimizes the potentiation action of serotonin at synaptic cleft, thus decreasing the risk of discontinuation symptoms.[34]
Synthesis

Currently, very few methods are used to synthesize (S)-dapoxetine. This novel approach consists of only six steps in which three main steps are shown above. The initial reactant is trans-cinnamyl alcohol, which is commercially available. Sharpless asymmetric epoxidation and Mitsunobu reaction have been used to produce expected (S)-dapoxetine. The overall yield is 35%. This method is considered a good choice compared to the known methods due to high yield and easily obtainable reactants.[35]
Regulatory history
Dapoxetine was created by Eli Lilly and in phase I clinical trial as an antidepressant. It never worked out well as a medication for the treatment of depression, though, and was shelved for a while before subsequently developed to treat PE. In December 2003, Eli Lilly sold the patent for dapoxetine to Pharmaceutical Product Development (PPD) for US$65 million. Eli Lilly may also receive royalties payment from PPD if the sale exceeds a certain amount. Research into the effectiveness of dapoxetine was revisited in 2020.[36]
ALZA is the current owner of dapoxetine, but PPD will receive milestone payments and drug royalties from ALZA. If approved, dapoxetine will be marketed in the US by Ortho McNeil pharmaceutical, Inc. Ortho McNeil and Janssen-Ortho Inc, or Janssen-Cilag are all units of Johnson & Johnson. As at 2005, dapoxetine was in phase III clinical trials, pending review by the FDA.[37]
Dapoxetine has been marketed and approved in more than 50 countries.[38] Dapoxetine has been approved in Italy, Spain, Mexico, South Korea, and New Zealand in 2009 and 2010; marketed in Sweden, Austria, Germany, Finland, Spain, Portugal, and Italy. It has also been approved in France, Russia, Malaysia, Philippines, Argentina, and Uruguay.[5]
See also
- Serotonin transporter
- Serotonergic system
- Serotonin receptors
References
- ↑ "Priligy (dapoxetine) Film-coated Tablets, for Oral Use. Full Prescribing Information" (in ru). Russian State Register of Medicines.. 25 July 2013. http://grls.rosminzdrav.ru/Grls_View_v2.aspx?idReg=86118&t=.
- ↑ 2.0 2.1 "Dapoxetine, a novel treatment for premature ejaculation, does not have pharmacokinetic interactions with phosphodiesterase-5 inhibitors". International Journal of Impotence Research 18 (1): 104–110. 2006. doi:10.1038/Sj.Ijir.3901420. PMID 16307008.
- ↑ "Dapoxetine: a new option in the medical management of premature ejaculation". Therapeutic Advances in Urology 4 (5): 233–251. October 2012. doi:10.1177/1756287212453866. PMID 23024705.
- ↑ "Priligy is used to Treat Premature Ejaculation" (in DE). 10 August 2015. https://deutschemedz.de/priligy/.
- ↑ 5.0 5.1 5.2 "Australian Public Assessment Report for Dapoxetine". Therapeutics Goods Administration. Australian Government. 2010. https://www.tga.gov.au/sites/default/files/auspar-priligy.pdf.
- ↑ 6.0 6.1 6.2 "Pharmacokinetic and pharmacodynamic features of dapoxetine, a novel drug for 'on-demand' treatment of premature ejaculation". BJU International 97 (2): 311–315. February 2006. doi:10.1111/J.1464-410x.2006.05911.X. PMID 16430636.
- ↑ "Dapoxetine: an evidence-based review of its effectiveness in treatment of premature ejaculation". Core Evidence 7: 1–14. 2012. doi:10.2147/CE.S13841. PMID 22315582.
- ↑ "Furiex Pharma gets rights to Priligy, some of which it sells on to Menarini". 15 May 2012. https://www.thepharmaletter.com/article/furiex-pharma-gets-rights-to-priligy-some-of-which-it-sells-on-to-menarini.
- ↑ "New agents in the treatment of premature ejaculation". Neuropsychiatric Disease and Treatment 2 (4): 489–503. December 2006. doi:10.2147/nedt.2006.2.4.489. PMID 19412497.
- ↑ 10.0 10.1 "Efficacy and tolerability of dapoxetine in treatment of premature ejaculation: an integrated analysis of two double-blind, randomised controlled trials". Lancet 368 (9539): 929–937. September 2006. doi:10.1016/s0140-6736(06)69373-2. PMID 16962882.
- ↑ "Dapoxetine has long-term efficacy in the treatment of premature ejaculation". Journal of Urology 175 (4): 297–298. 2006. doi:10.1016/S0022-5347(18)33154-9.
- ↑ "AUA guideline on the pharmacologic management of premature ejaculation". The Journal of Urology 172 (1): 290–294. July 2004. doi:10.1097/01.ju.0000132159.61156.ea. PMID 15201797.
- ↑ "Dapoxetine: An Innovative Approach in the Therapeutic Management In Animal Model of Depression". Pakistan Journal of Pharmaceutical Research 2 (1): 15–22. January 2016. doi:10.22200/pjpr.2016115-22. https://www.researchgate.net/publication/301200771.
- ↑ "Antistress and antidepressant properties of dapoxetine and vortioxetine". Acta Neurobiologiae Experimentalis 80 (3): 217–224. 2020. doi:10.21307/ane-2020-020. PMID 32990281.
- ↑ "Dapoxetine for the treatment of premature ejaculation: results from a randomized, double-blind, placebo-controlled phase 3 trial in 22 countries". European Urology 55 (4): 957–967. April 2009. doi:10.1016/J.Eururo.2009.01.025. PMID 19195772.
- ↑ "Éjaculation précoce: pas de dapoxétine" (in fr). Prescrire 29 (313): 811–814. 2009. https://www.prescrire.org/fr/3/31/48533/0/NewsDetails.aspx.
- ↑ "Discontinuation of Dapoxetine Treatment in Patients With Premature Ejaculation: A 2-Year Prospective Observational Study.". The Journal of Sexual Medicine 5 (2): 99–105. June 2017. doi:10.1016/j.esxm.2017.02.003. PMID 28395997.
- ↑ "Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients. Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction". The Journal of Clinical Psychiatry 62 (Suppl 3): 10–21. 2001. PMID 11229449.
- ↑ "Dapoxetine-A Novel Drug for Premature Ejaculation". Delhi Psychiatry Journal 14 (1): 5. 2011.
- ↑ 20.0 20.1 "Dapoxetine for the treatment of premature ejaculation: Lack of interaction with ethanol". Journal of Urology 173 (4): 239. 2005. doi:10.1016/S0022-5347(18)35971-8.
- ↑ "Monoaminergic transporter binding and inhibition profile of dapoxetine, a medication for the treatment of premature ejaculation". Journal of Urology 173 (4): 239. 2005.
- ↑ "The Central Mechanisms of Sexual Function". Boston University School of Medicine. 17 November 2003. http://www.bumc.bu.edu/sexualmedicine/publications/the-central-mechanisms-of-sexual-function/.
- ↑ "Physiology of ejaculation: emphasis on serotonergic control". European Urology 48 (3): 408–417. September 2005. doi:10.1016/j.eururo.2005.05.017. PMID 15996810.
- ↑ "Supraspinal site of action for the inhibition of ejaculatory reflex by dapoxetine". European Urology 51 (3): 825–832. March 2007. doi:10.1016/J.Eururo.2006.10.011. PMID 17064843.
- ↑ "Efficacy and safety of dapoxetine for the treatment of premature ejaculation: integrated analysis of results from five phase 3 trials". The Journal of Sexual Medicine 8 (2): 524–539. February 2011. doi:10.1111/j.1743-6109.2010.02097.x. PMID 21059176.
- ↑ "Dapoxetine: a guide to its use in premature ejaculation". Drugs & Therapy Perspectives 27 (2): 1–4. February 2011. doi:10.2165/11206280-000000000-00000.
- ↑ "Pharmacokinetics of single and multiple escalating doses of dapoxetine in healthy volunteers". Clinical Pharmacology & Therapeutics 75 (2): P32. 2004. doi:10.1016/J.Clpt.2003.11.123.
- ↑ "Cardiovascular safety profile of dapoxetine during the premarketing evaluation". Drugs in R&D 11 (1): 1–11. 2011. doi:10.2165/11587660-000000000-00000. PMID 21410293.
- ↑ "Suicide rates in clinical trials of SSRIs, other antidepressants, and placebo: analysis of FDA reports". The American Journal of Psychiatry 160 (4): 790–792. April 2003. doi:10.1176/Appi.Ajp.160.4.790. PMID 12668373.
- ↑ "Selective serotonin reuptake inhibitor discontinuation syndrome: a review". Advances in Therapy 19 (1): 17–26. 2002. doi:10.1007/Bf02850015. PMID 12008858.
- ↑ "Dapoxetine: a new option in the medical management of premature ejaculation". Therapeutic Advances in Urology 4 (5): 233–251. October 2012. doi:10.1177/1756287212453866. PMID 23024705.
- ↑ "Emerging treatments for premature ejaculation: focus on dapoxetine". Neuropsychiatric Disease and Treatment 5: 37–46. 2009. doi:10.2147/ndt.s3251. PMID 19557098.
- ↑ "Dapoxetine: a novel treatment for premature ejaculation". Drugs of Today 45 (9): 669–678. September 2009. doi:10.1358/dot.2009.45.9.1388694. PMID 19956808.
- ↑ "Premature ejaculation: definition and drug treatment". Drugs 67 (4): 547–568. 2007. doi:10.2165/00003495-200767040-00005. PMID 17352514.
- ↑ "Stereoselective synthesis of (S)-dapoxetine starting from trans-cinnamyl alcohol". Arkivoc 2008 (16): 302–310. 2008. doi:10.3998/ark.5550190.0009.g28.
- ↑ "Antistress and antidepressant properties of dapoxetine and vortioxetine". Acta Neurobiologiae Experimentalis 80 (3): 217–224. 2020. doi:10.21307/ane-2020-020. PMID 32990281.
- ↑ "Dapoxetine: LY 210448". Drugs in R&D 6 (5): 307–311. 2005. doi:10.2165/00126839-200506050-00007. PMID 16128601.
- ↑ "Medical Non-Endocrine-Targeted Therapies: Ejaculatory Dysfunction and Immunotherapy". Encyclopedia of Reproduction (2nd ed.). 2018. pp. 324–327. doi:10.1016/B978-0-12-801238-3.64785-2. ISBN 9780128151457.
{{Navbox
| name = Sexual dysfunction pharmacotherapies | title = Sexual dysfunction pharmacotherapies | state = autocollapse | bodyclass = hlist | listclass = hlist
| group1 = Dopamine agonists | list1 =
- Apomorphine
- Cabergoline
- Lisuride
- Pergolide
- Piribedil
- Pramipexole
- Quinagolide
- Ropinirole
- Rotigotine
- Terguride
| group2 = Melanocortin agonists | list2 =
| group3 = PDE5 inhibitors | list3 =
- Acetildenafil
- Aildenafil
- Avanafil
- Icariin
- Lodenafil
- Mirodenafil
- Nitrosoprodenafil
- Sildenafil
- Sulfoaildenafil
- Tadalafil
- Udenafil
- Vardenafil
| group4 = Sex steroids | list4 =
- Androgens (e.g., [[testosterone, methyltestosterone, other anabolic steroids)
- Estrogens (e.g., [[estradiol, ethinylestradiol, conjugated equine estrogens (Premarin))
- Progestogens (e.g., [[progesterone, progestins)
- Mixed (e.g., tibolone)
| group5 = Others | list5 =
- Afrodor (acecarbromal, quebracho, vitamin E)
- Alkyl nitrites
- Alprostadil
- Amantadine
- Bupropion
- Buspirone
- Cyproheptadine
- Dapoxetine
- Flibanserin
- Mirtazapine
- Moxisylyte
- Oxytocin
- Papaverine
- Phentolamine
- Psychostimulants (e.g., amphetamines, cocaine, methylphenidate)
- Rauwolscine (Rauvolfia)
- Trazodone
- Yohimbine (Yohimbe)
| below =
}}
 |

