Chemistry:Desmethylsertraline
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 66 hours |
| Excretion | urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
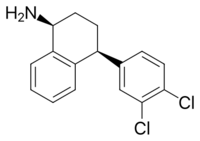
| Formula | C16H15Cl2N |
| Molar mass | 292.20 g·mol−1 |
| 3D model (JSmol) | |
| |
Desmethylsertraline (DMS), also known as norsertraline, is an active metabolite of the antidepressant drug sertraline. Like sertraline, desmethylsertraline acts as a monoamine reuptake inhibitor, and may be responsible for some of its parent's therapeutic benefits; however, the effects of DMS's main activity of increasing serotonin levels via binding to the serotonin transporter appears to be negligible as in vivo testing showed no measurable change in brain activity despite a nearly 20-fold increase in DMS blood levels compared to the EC50 (i.e. the amount required to achieve the desired effect in 50% of the population) of its parent drug sertraline.[1] DMS is significantly less potent relative to sertraline as a serotonin reuptake inhibitor (Ki = 76 nM vs. 3 nM, respectively), but conversely, is more balanced as a monoamine reuptake inhibitor (5-HT (Ki) = 76 nM; NE (Ki) = 420 nM; DA (Ki) = 440 nM), which has the effective result of DMS contrarily behaving as a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI), with about 5.5-fold preference for inhibiting serotonin reuptake relative to catecholamine reuptake.[2]
Dasotraline, a stereoisomer of DMS, is also an SNDRI, and has been investigated for the potential clinical treatment of major depressive disorder, attention-deficit disorder, and eating disorders, but has not been approved or marketed for any indication.
See also
- Desmethylcitalopram
- Desmethylvenlafaxine
- Didesmethylcitalopram
- Indatraline
- Lometraline
- Norfluoxetine
- Tametraline
References
- ↑ "Comparison of the effects of sertraline and its metabolite desmethylsertraline on blockade of central 5-HT reuptake in vivo". Neuropsychopharmacology 14 (4): 225–231. April 1996. doi:10.1016/0893-133X(95)00112-Q. PMID 8924190.
- ↑ "Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication". Life Sciences 57 (5): 411–441. 1995. doi:10.1016/0024-3205(95)00209-o. PMID 7623609.
 |

