Chemistry:PPPA (drug)
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
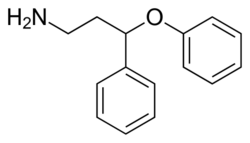
| Formula | C15H17NO |
| Molar mass | 227.307 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
PPPA, or 3-phenoxy-3-phenylpropan-1-amine, is a drug which is described as an antidepressant.[1] It was derived by Eli Lilly from the antihistamine diphenhydramine, a diphenylmethane derivative with additional properties as a selective serotonin reuptake inhibitor (SSRI), and has been the basis for the subsequent discovery of a number of other antidepressant drugs.[2][3][4]
List of PPPA derivatives
- Atomoxetine ((3R)-N-methyl-3-(2-methylphenoxy)-3-phenylpropan-1-amine) — NRI[1]
- Fluoxetine (N-methyl-3-(4-(trifluoromethyl)phenoxy)-3-phenylpropan-1-amine) — SSRI[2]
- N-Methyl-PPPA (N-methyl-3-phenoxy-3-phenylpropan-1-amine) — SNRI[2][4]
- Nisoxetine (N-methyl-3-(2-methoxyphenoxy)-3-phenylpropan-1-amine) — NRI[1]
- Norfluoxetine (3-(4-(trifluoromethyl)phenoxy)-3-phenylpropan-1-amine) — SSRI[3]
- Seproxetine ((S)-3-(4-(trifluoromethyl)phenoxy)-3-phenylpropan-1-amine) — SSRI[5]
Structurally related drugs include dapoxetine, duloxetine, edivoxetine, femoxetine, paroxetine, reboxetine, and viloxazine, all of which act, similarly, as monoamine reuptake inhibitors, and most of which are, again similarly, antidepressants.[1][3]
Zimelidine is an antidepressant and SSRI which was derived from the antihistamine pheniramine, which, similarly to its analogues brompheniramine and chlorpheniramine, possesses SNRI properties.[4] Fluvoxamine, another antidepressant and SSRI, was developed from the antihistamine tripelennamine, which possesses SNDRI actions.[6]
See also
References
- ↑ 1.0 1.1 1.2 1.3 "Serotonin Receptors and Drugs Affecting Serotonergic Neurotransmission". Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. 2008. pp. 414–. ISBN 978-0-7817-6879-5. https://books.google.com/books?id=R0W1ErpsQpkC&pg=PA414.
- ↑ 2.0 2.1 2.2 "Contribution of Pharmacology to Development of Monoaminergic Hypotheses of Depression". Neurobiology of Depression. CRC Press. 9 September 2011. pp. 132–. ISBN 978-1-4398-3850-1. https://books.google.com/books?id=lmXRBQAAQBAJ&pg=PA132.
- ↑ 3.0 3.1 3.2 "Selective Serotonin Reuptake Inhibitors for the Treatment of Depression". Analogue-based Drug Discovery II. John Wiley & Sons. 24 August 2010. pp. 35, 282, 284. ISBN 978-3-527-63212-1. https://books.google.com/books?id=h2Kd8ci4Ln8C&pg=PA282.
- ↑ 4.0 4.1 4.2 "Drugs Originating from the Screening of Organic Chemicals". Drug Discovery: A History. John Wiley & Sons. 31 October 2005. pp. 416–417. ISBN 978-0-470-01552-0. https://books.google.com/books?id=jglFsz5EJR8C&pg=PA416.
- ↑ "CNS stimulants and CNS-active drugs affecting the serotonergic system". Pharmaceutical Chemistry. Elsevier Health Sciences. 9 February 2011. pp. 1061–. ISBN 978-0-7020-4850-0. https://books.google.com/books?id=-lQM4xHjWeUC&pg=PT1061.
- ↑ Let Them Eat Prozac: The Unhealthy Relationship Between the Pharmaceutical Industry and Depression. NYU Press. 1 June 2004. pp. 295–. ISBN 978-0-8147-7300-0. https://books.google.com/books?id=5w64WC_-jbMC&pg=PA295.
Further reading
- "Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication". Life Sciences 57 (5): 411–441. 1995. doi:10.1016/0024-3205(95)00209-o. PMID 7623609.
 |

