Chemistry:Lortalamine
From HandWiki
Short description: Chemical compound
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Pharmacokinetic data | |
| Elimination half-life | 5 hours |
| Excretion | Renal (98%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
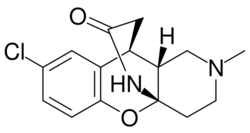
| Formula | C15H17ClN2O2 |
| Molar mass | 292.76 g·mol−1 |
| 3D model (JSmol) | |
| |
Lortalamine (LM-1404) is an antidepressant which was synthesized in the early 1980s.[1][2] It acts as a potent and highly selective norepinephrine reuptake inhibitor.[3][4] Lortalamine was under development for clinical use but was shelved, likely due to the finding that it produced ocular toxicity in animals.[5][6] It has been used to label the norepinephrine transporter in positron emission tomography studies.[4][7][8]
See also
References
- ↑ David J. Triggle (1997). Dictionary of pharmacological agents. London: Chapman & Hall. ISBN 0-412-46630-9. https://books.google.com/books?id=A0THacd46ZsC&q=lortalamine&pg=PA1235.
- ↑ "Plasma levels, elimination and metabolic fate of 4a-amino-8-chloro-2-methyl-1,2,3,4,4a,10a-hexahydro-10H-benzopyrano[3,2-cpyridin-10-ylacetic acid lactam, a new antidepressive agent, in rats and dogs"]. Drug Metabolism and Disposition 9 (3): 233–9. 1981. PMID 6113932. http://dmd.aspetjournals.org/cgi/pmidlookup?view=long&pmid=6113932.
- ↑ "Pharmacology of lortalamine, a new potent non-tricyclic antidepressant". Arzneimittel-Forschung 35 (11): 1655–62. 1985. PMID 4091869.
- ↑ 4.0 4.1 "Synthesis and C-11 labeling of three potent norepinephrine transporter selective ligands ((R)-nisoxetine, lortalamine, and oxaprotiline) for comparative PET studies in baboons". Bioorganic & Medicinal Chemistry 13 (15): 4658–66. August 2005. doi:10.1016/j.bmc.2005.04.062. PMID 15914010. https://zenodo.org/record/1258810.
- ↑ "Metabolism of the anti-depressant lortalamine". European Journal of Drug Metabolism and Pharmacokinetics 10 (3): 209–15. 1985. doi:10.1007/bf03189744. PMID 4085522.
- ↑ "Ocular toxicity in beagle dogs with lortalamine, a non tricyclic antidepressant compound". Drug and Chemical Toxicology 13 (4): 309–23. 1990. doi:10.3109/01480549009032289. PMID 2279460.
- ↑ "Comparative evaluation of positron emission tomography radiotracers for imaging the norepinephrine transporter: (S,S) and (R,R) enantiomers of reboxetine analogs ([11C]methylreboxetine, 3-Cl-[11C]methylreboxetine and [18F]fluororeboxetine), (R)-[11C]nisoxetine, [11C]oxaprotiline and [11C]lortalamine". Journal of Neurochemistry 94 (2): 337–51. July 2005. doi:10.1111/j.1471-4159.2005.03202.x. PMID 15998285.
- ↑ "PET imaging of norepinephrine transporters". Current Pharmaceutical Design 12 (30): 3831–45. 2006. doi:10.2174/138161206778559687. PMID 17073682. https://zenodo.org/record/1235852.
 |
