Chemistry:GBR-12935
From HandWiki
Short description: Chemical compound
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
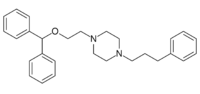
| Formula | C28H34N2O |
| Molar mass | 414.593 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
GBR-12935 is a piperazine derivative which is a potent and selective dopamine reuptake inhibitor. It was originally developed in its 3H radiolabelled form for the purpose of mapping the distribution of dopaminergic neurons in the brain by selective labelling of dopamine transporter proteins.[1] This has led to potential clinical uses in the diagnosis of Parkinson's disease,[2] although selective radioligands such as Ioflupane (¹²³I) are now available for this application. GBR-12935 is now widely used in animal research into Parkinson's disease and the dopamine pathways in the brain.[3][4][5]
See also
References
- ↑ "[3H]GBR-12935: a specific high affinity ligand for labeling the dopamine transport complex". European Journal of Pharmacology 107 (2): 289–90. January 1985. doi:10.1016/0014-2999(85)90075-5. PMID 3979428.
- ↑ "[3H]GBR-12935 binding to the dopamine transporter is decreased in the caudate nucleus in Parkinson's disease". Journal of Neurochemistry 49 (2): 617–21. August 1987. doi:10.1111/j.1471-4159.1987.tb02908.x. PMID 3598589.
- ↑ "Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex". Behavioural Brain Research 148 (1–2): 107–17. January 2004. doi:10.1016/s0166-4328(03)00190-6. PMID 14684252.
- ↑ "Dopamine transporter blockade increases LTP in the CA1 region of the rat hippocampus via activation of the D3 dopamine receptor". Learning & Memory 13 (2): 161–7. 2006. doi:10.1101/lm.63806. PMID 16585791.
- ↑ "Design and synthesis of 2- and 3-substituted-3-phenylpropyl analogs of 1-[2-[bis(4-fluorophenyl)methoxyethyl]-4-(3-phenylpropyl)piperazine and 1-[2-(diphenylmethoxy)ethyl]-4-(3-phenylpropyl)piperazine: role of amino, fluoro, hydroxyl, methoxyl, methyl, methylene, and oxo substituents on affinity for the dopamine and serotonin transporters"]. Journal of Medicinal Chemistry 51 (9): 2795–806. May 2008. doi:10.1021/jm701270n. PMID 18393401.
 |

