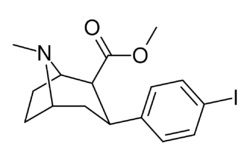
Chemistry:RTI-55
 | |
| Clinical data | |
|---|---|
| Other names | (–)-2β-Carbomethoxy-3β-(4-iodophenyl)tropane; β-CIT; iometopane; iometopane I 123 (USAN); iometopane 123I (INN); iometopane I 125 (USAN); iometopane 125I (INN) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C16H20INO2 |
| Molar mass | 385.245 g·mol−1 |
| 3D model (JSmol) | |
| |
| (verify) | |
RTI(-4229)-55, also called RTI-55 or iometopane, is a phenyltropane-based psychostimulant used in scientific research and in some medical applications. This drug was first cited in 1991.[1] RTI-55 is a non-selective dopamine reuptake inhibitor derived from methylecgonidine. However, more selective analogs are derived by conversion to "pyrrolidinoamido" RTI-229, for instance. Due to the large bulbous nature of the weakly electron withdrawing iodo halogen atom, RTI-55 is the most strongly serotonergic of the simple para-substituted troparil based analogs.[2] In rodents RTI-55 actually caused death at a dosage of 100 mg/kg, whereas RTI-51 and RTI-31 did not.[2] Another notable observation is the strong propensity of RTI-55 to cause locomotor activity enhancements,[2] although in an earlier study, RTI-51 was actually even stronger than RTI-55 in shifting baseline LMA.[3] This observation serves to highlight the disparities that can arise between studies.
RTI-55 is one of the most potent phenyltropane stimulants commercially available, which limits its use in humans, as it might have significant abuse potential if used outside a strictly controlled medical setting.[4] However, it is definitely worthy of mentioning that increasing the size of the halogen atom attached to troparil serves to reduce the number of lever responses in a session when these analogs were compared in a study.[5] Although RTI-55 wasn't specifically examined in this study the number of lever responses in a given session was of the order cocaine > WIN35428 > RTI-31 > RTI-51.
In contrast to RTI-31 which is predominantly dopaminergic, increasing the size of the covalently bonded halogen from a chlorine to an iodine markedly increases the affinity for the SERT, while retaining mostly all of its DAT blocking activity.
The radiopharmaceutical forms of RTI-55, in which the iodine atom is radioiodine so that the drug can be used in single-photon emission computed tomography, are called iometopane I 123 (USAN) or iometopane 123I (INN) and iometopane I 125 (USAN) or iometopane 125I (INN). The 123I and 125I isotopes are favored because they are very-high-energy γ-ray emitters.
Compared to the "WIN" compounds, extremely low Ki values are attainable.
Uses
RTI-55 is mainly used in scientific research into the dopamine reuptake transporter. Various radiolabelled forms of RTI-55 (with different radioactive isotopes of iodine used depending on the application) are used in both humans and animals to map the distribution of dopamine transporters and serotonin transporters in the brain.[6][7] The 123I derivative is known as iometopane.
The main practical application for this drug in medicine is to assess the rate of dopamine neuron degradation in the brains of sufferers of Parkinson's disease,[8][9] and some other conditions such as progressive supranuclear palsy.[10]
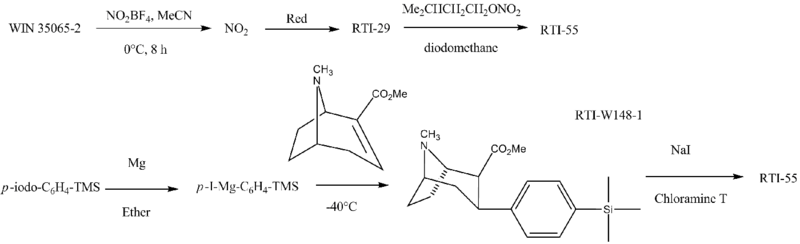
Chemistry
RTI-55 is made as follows:[11][12][13]
See also
References
- ↑ "[125IRTI-55: a potent ligand for dopamine transporters"]. European Journal of Pharmacology 194 (1): 133–4. February 1991. doi:10.1016/0014-2999(91)90137-F. PMID 2060590. https://zenodo.org/record/1253870.
- ↑ 2.0 2.1 2.2 "Monoamine transporter binding, locomotor activity, and drug discrimination properties of 3-(4-substituted-phenyl)tropane-2-carboxylic acid methyl ester isomers". Journal of Medicinal Chemistry 47 (25): 6401–9. December 2004. doi:10.1021/jm0401311. PMID 15566309.
- ↑ "Locomotor stimulant effects of novel phenyltropanes in the mouse". Drug and Alcohol Dependence 65 (1): 25–36. December 2001. doi:10.1016/S0376-8716(01)00144-2. PMID 11714587.
- ↑ "Reinforcing and discriminative stimulus effects of beta-CIT in rhesus monkeys". Pharmacology, Biochemistry, and Behavior 51 (4): 953–6. August 1995. doi:10.1016/0091-3057(95)00032-r. PMID 7675883.
- ↑ "A reduced rate of in vivo dopamine transporter binding is associated with lower relative reinforcing efficacy of stimulants". Neuropsychopharmacology 31 (2): 351–62. February 2006. doi:10.1038/sj.npp.1300795. PMID 15957006.
- ↑ "In vivo imaging of dopamine reuptake sites in the primate brain using single photon emission computed tomography (SPECT) and iodine-123 labeled RTI-55". Synapse 10 (2): 169–72. February 1992. doi:10.1002/syn.890100210. PMID 1585258.
- ↑ "Displacement of serotonin and dopamine transporters by venlafaxine extended release capsule at steady state: a [123I]2beta-carbomethoxy-3beta-(4-iodophenyl)-tropane single photon emission computed tomography imaging study". Journal of Clinical Psychopharmacology 27 (1): 71–5. February 2007. doi:10.1097/JCP.0b013e31802e0017. PMID 17224717.
- ↑ "[123I] beta-CIT binding and SPET compared with clinical diagnosis in parkinsonism". Nuclear Medicine Communications 21 (5): 417–24. May 2000. doi:10.1097/00006231-200005000-00002. PMID 10874697.
- ↑ "Optimized, automated striatal uptake analysis applied to SPECT brain scans of Parkinson's disease patients". Journal of Nuclear Medicine 48 (6): 857–64. June 2007. doi:10.2967/jnumed.106.037432. PMID 17504864.
- ↑ "Topography of dopamine transporter availability in progressive supranuclear palsy: a voxelwise [123I]beta-CIT SPECT analysis". Archives of Neurology 63 (8): 1154–60. August 2006. doi:10.1001/archneur.63.8.1154. PMID 16908744.
- ↑ U.S. Patent 5,128,118
- ↑ U.S. Patent 6,123,917
- ↑ "3 Beta-(p-trimethylsilylphenyl)tropane-2 beta-carboxylic acid methyl ester: a new precursor for the preparation of [123I]RTI-55". Applied Radiation and Isotopes 47 (1): 79–81. January 1996. doi:10.1016/0969-8043(95)00259-6. PMID 8589674.
 |