Chemistry:Mazindol
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Mazanor, Sanorex |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 93% |
| Metabolism | Hepatic |
| Elimination half-life | 10–13 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C16H13ClN2O |
| Molar mass | 284.74 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
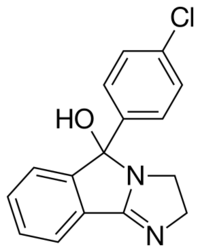
Mazindol (brand names Mazanor, Sanorex) is a stimulant drug which is used as an appetite suppressant.[1] It was developed by Sandoz-Wander in the 1960s.[2]
Medical uses
Mazindol is used in short-term (i.e., a few weeks) treatment of obesity, in combination with a regimen of weight reduction based on caloric restriction, exercise, and behavior modification in people with a body mass index greater than 30, or in those with a body mass index greater than 27 in the presence of risk factors such as hypertension, diabetes, or hyperlipidemia. Mazindol is not currently available as a commercially marketed and FDA-regulated prescription agent for the treatment of obesity.
There is a Swiss study investigating its efficacy in treating ADHD.[3]
Additional patented uses include for the treatment of schizophrenia,[4] reducing cravings for cocaine,[5] and for the treatment of neurobehavioral disorders.[6]
Pharmacology
| Site | Ki (nM) |
|---|---|
| DAT | 25.9 |
| NET | 2.88 |
| SERT | 272 |
Mazindol is a sympathomimetic amine, which is similar to amphetamine. It stimulates the central nervous system, which increases heart rate and blood pressure, and decreases appetite. Sympathomimetic anoretics (appetite suppressants) are used in the short-term treatment of obesity. Their appetite-reducing effect tends to decrease after a few weeks of treatment. Because of this, these medicines are useful only during the first few weeks of a weight-loss program.
Although the mechanism of action of the sympathomimetics in the treatment of obesity is not fully known, these medications have pharmacological effects similar to those of amphetamines. Like other sympathomimetic appetite suppressants, mazindol is thought to act as a reuptake inhibitor of norepinephrine. In addition, it inhibits dopamine and serotonin reuptake. The recommended dosage is 2 mg per day for 90 days in patients 40 kg overweight and under; 4 mg a day in patients more than 50 kg overweight; divided into two doses separated by a 12-hour window between each dose.
Overdose
Symptoms of a mazindol overdose include: restlessness, tremor, rapid breathing, confusion, hallucinations, panic, aggressiveness, nausea, vomiting, diarrhea, an irregular heartbeat, and seizures.
Analogues
An analogue of mazindol was reported that was stated to be less toxic than the parent drug from which it was derived.[8] It is made from Chemrat (Pindone).
QSAR Dialog

From available QSAR data, the following trends are apparent:[10]
- Desoxylation of the tertiary alcohol in mazindol improves DAT and SERT binding without substantially reducing NET affinity. This compound has been called "Mazindane".[11]
- Removal of the p-chlorine atom from the phenyl ring of mazindol increases NET affinity and substantially reduces DAT and SERT affinity.
- Expansion of the imidazoline ring system in mazindol to the corresponding six-membered homolog increases DAT affinity by ~10 fold.
- Replacement of the phenyl moiety with a naphthyl ring system results in a ~50 fold increase in SERT affinity without significant decreases in NET or DAT affinities.
- Halogenation of 3' and/or 4' position of the phenyl ring of mazindol results in increased potency at NET, DAT, and SERT.
- Fluorination of the 7' position of the tricyclic phenyl ring results in a ~2 fold increase in binding affinity to the DAT.
| Compound | S. Singh's alphanumeric assignation (name) |
R | R′ | R′′ | IC50 (nM) (Inhibition of [3H]WIN 35428 binding) |
IC50 (nM) (Inhibition of [3H]DA uptake) |
Selectivity uptake/binding |
|---|---|---|---|---|---|---|---|
| (cocaine) | 89.1 ± 8 | 208 ± 12 | 2.3 | ||||

| |||||||
| (mazindol) | H | H | 4′-Cl | 8.1 ± 1.2 | 8.4 ± 1.3 | 1.0 | |
| 384a | H | H | H | 66.0 ± 8.9 | 124 ± 37 | 1.9 | |
| 384b | H | H | 4′-F | 13.3 ± 1.8 | 25.4 ± 2.7 | 1.9 | |
| 384c | H | 7-F | H | 29.7 ± 7.0 | 78 ± 46 | 2.6 | |
| 384d | H | H | 2′-Cl | 294 ± 6 | 770 ± 159 | 2.6 | |
| 384e | H | H | 3′-Cl | 4.3 ± 0.4 | 9.2 ± 5.3 | 2.1 | |
| 384f | CH3 | H | 4′-Cl | 50.4 ± 5.5 | 106 ± 5.6 | 2.1 | |
| 384g | H | 6-Cl | H | 57.2 ± 8.3 | 58 ± 6.4 | 1.0 | |
| 384h | H | 7-Cl | H | 85.4 ± 14 | 55.17 | 0.6 | |
| 384i | H | 7-F | 4′-Cl | 6.5 ± 1.2 | 15 ± 9 | 2.3 | |
| 384j | H | 7-Cl | 4′-F | 52.8 ± 8.7 | 53 ± 18 | 1.0 | |
| 384k | H | H | 2′,4′-Cl2 | 76.5 ± 1.11 | 92 ± 19 | 1.2 | |
| 384l | H | H | 3′,4′-Cl2 | 2.5 ± 0.5 | 1.4 ± 1.6 | 0.6 | |
| 384m | H | 7,8-Cl2 | 4′-Cl | 13.6 ± 1.5 | |||
| 384n | H | H | 2′-Br | 1340 ± 179 | |||
| 384o | H | H | 4′-Br | 2.6 ± 1.5 | 8.6 ± 3.5 | 3.3 | |
| 384p | H | H | 4′-I | 17.2 ± 0.9 | 14 ± 6.4 | 0.8 |
| Compound | S. Singh's alphanumeric assignation (name) |
R | R′ | IC50 (nM) (Inhibition of [3H]WIN 35428 binding) |
IC50 (nM) (Inhibition of [3H]DA uptake) |
Selectivity uptake/binding |
|---|---|---|---|---|---|---|

| ||||||
| 388a | H | H | 5.8 ± 1.6 | 18 ± 11 | 3.1 | |
| 388b | H | 2′-F | 23.2 ± 1.7 | 89 ± 2.8 | 3.8 | |
| 388c | H | 3′-F | 2.0 ± 0.02 | 3.1 ± 1.8 | 1.6 | |
| 388d | H | 4′-F | 3.2 ± 1.7 | 8.5 ± 4.9 | 0.4 | |
| 388e | H | 3′-Cl | 1.0 ± 0.2 | 1.3 ± 0.14 | 1.3 | |
| 388f | H | 4′-Cl | 1.7 ± 0.2 | 1.4 ± 0.35 | 0.8 | |
| 388g | CH3 | 4′-Cl | 6.3 ± 4.5 | 1.7 ± 1.6 | 0.3 | |
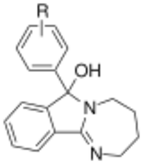
| ||||||
| 389a | H | 5.9 ± 0.1 | 11 ± 3.2 | 2.0 | ||
| 389b | 4′-Cl | 1.5 ± 0.1 | 3.4 ± 2.3 | 2.3 | ||
| 389c | 3′,4′-Cl2 | 1.7 ± 0.1 | 0.26 ± 0.16 | 0.2 |
| Structure | n | R | R' | R" | hSERT | hNET | hDAT | SERT/DAT Selectivity |
NET/DAT Selectivity |
|---|---|---|---|---|---|---|---|---|---|
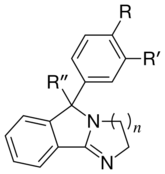
| |||||||||
| 1 | Cl | H | OH | 94 ± 32 | 4.9 ± 0.5 | 43 ± 20 | 2.2 | 0.1 | |
| 1 | Cl | H | H | 15 ± 5 | 6.9 ± 1.5 | 6.0 ± 0.7 | 2.5 | 1.2 | |
| 1 | H | H | OH | 2140 ± 450 | 2.8 ± 0.92 | 730 ± 180 | 2.9 | 0.004 | |
| 1 | Naphthyl | OH | 1.8 ± 1.3 | 4.5 ± 1.5 | 66 ± 10 | 0.03 | 0.07 | ||
| 2 | Cl | H | OH | 53 ± 7 | 4.9 ± 0.5 | 3.7 ± 0.4 | 14.3 | 1.3 | |
| 2 | OH | H | OH | 60 ± 19 | 1.9 ± 0.15 | 59.0 ± 3.6 | 1 | 0.03 | |
| 2 | OMe | H | OH | 94 ± 34 | 4.1 ± 1.4 | 30.4 ± 2.4 | 3.1 | 0.1 | |
| 2 | -OCH2O- | OH | 83 ± 29 | 0.62 ± 0.25 | 2.21 ± 0.3 | 37.7 | 0.3 | ||
Chemistry
Tautomers
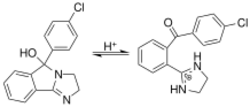
Mazindol exhibits pH dependent tautomerization between the keto form and the cyclic hemiaminal. Mazindol exists in the tricyclic (-ol) form in neutral media and undergoes protonation to the benzophenone tautomer in acidic media. QSAR studies have indicated that the ability of mazindol to inhibit NE and DA reuptake may be mediated by the protonated (benzophenone) tautomer.[12]
Synthesis
The precursor for mazindol was described in the synthesis of Chlortalidone.
The synthesis of mazindol starts by reaction of a substituted benzoylbenzoic acid (1) with ethylenediamine. The product 3 can be rationalized as being an aminal from the initially formed monoamide 2. This is then subjected to reduction with LiAlH4 and-without isolation-air oxidation. Reduction probably proceeds to the mixed aminal/carbinolamine 4; such a product would be expected to be in equilibrium with the alternate aminal 5. The latter would be expected to predominate because of the greater stability of aldehyde aminals over the corresponding ketone derivatives. Air oxidation of the tetrahydroimidazole to the imidazoline will then remove 5 from the equilibrium. There is thus obtained the anorectic agent mazindol (6). The synthesis of homomazindol (the six-member ring A homologue) is accomplished by substitution of 1,2-diaminoethane with 1,3-diaminopropane.
An alternative synthesis was described:
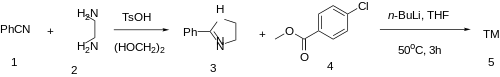
2-Phenyl-2-Imidazoline [936-49-2] (3) Methyl 4-Chlorobenzoate [1126-46-1] (4)
Research
As of 2016 mazindol was being studied in clinical trials for attention-deficit hyperactivity disorder.[17]
See also
Notes
References
- ↑ "Pharmacology and biochemical profile of a new anorectic drug: mazindol". Cent. Mech. Anorectic Drugs: 145–64. 1978.
- ↑ 2.0 2.1 Houlihan WJ, Eberle MK, "1H-Isoindole Intermediates", US patent granted 3597445, issued 3 August 1971, assigned to Sandoz AG
- ↑ "Swiss biotech NLS Pharma's ADHD drug succeeds in mid-stage study" (in en). Reuters. 2017-05-31. https://www.reuters.com/article/us-nls-pharma-study-adhd-idUSKBN18R0E4.
- ↑ "Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia" US patent 5447948, issued 5 September 1995
- ↑ Berger SP, "Dopamine uptake inhibitors in reducing substance abuse and/or craving", US patent 5217987, issued 8 June 1993
- ↑ Kovacs B, Pinegar L, "se of isoindoles for the treatment of neurobehavioral disorders", WO patent 2009155139, published 23 December 2009
- ↑ "Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin". Synapse 39 (1): 32–41. January 2001. doi:10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. PMID 11071707.
- ↑ "Analogs of the anorexic mazindol". Journal of Pharmaceutical Sciences 64 (8): 1375–8. August 1975. doi:10.1002/jps.2600640824. PMID 1151711.
- ↑ 9.0 9.1 9.2 "Chemistry, design, and structure-activity relationship of cocaine antagonists". Chemical Reviews 100 (3): 925–1024. March 2000. doi:10.1021/cr9700538. PMID 11749256. https://www.erowid.org/archive/rhodium/pdf/cocaineanalogs.pdf.
- ↑ 10.0 10.1 10.2 "Benzo- and cyclohexanomazindol analogues as potential inhibitors of the cocaine binding site at the dopamine transporter". Journal of Medicinal Chemistry 45 (19): 4110–8. September 2002. doi:10.1021/jm010301z. PMID 12213054."Mazindol analogues as potential inhibitors of the cocaine binding site at the dopamine transporter". Journal of Medicinal Chemistry 45 (19): 4097–109. September 2002. doi:10.1021/jm010302r. PMID 12213053.
- ↑ "Assessment of mazindane, a pro-drug form of mazindol, in assays used to define cocaine treatment agents". European Journal of Pharmacology 458 (3): 263–73. January 2003. doi:10.1016/s0014-2999(02)02791-7. PMID 12504782.
- ↑ "Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain". The Journal of Pharmacology and Experimental Therapeutics 199 (3): 649–661. December 1976. PMID 994022.
- ↑ "5-aryl-2,3-dihydro-5H-imidazo[2,1-a]isoindol-5-ols. A novel class of anorectic agents". Journal of Medicinal Chemistry 18 (2): 177–82. February 1975. doi:10.1021/jm00236a014. PMID 804553.
- ↑ "Improvements in or Relating to Imidazoisoindole Derivatives" DE patent granted 1814540, issued 3 July 1969, assigned to Sandoz AG
- ↑ Houlihan WJ, Eberle MK, "Heterocyclische Verbindungen und Verfahren zu ihrer Herstellung", DE patent granted 1930488, issued 19 March 1970, assigned to Sandoz AG
- ↑ "Midazolinyl Phenyl Carbonyl Acid Addition Salts and Related Compounds" US patent granted 3763178, issued 2 October 1973, assigned to American Home Products
- ↑ "Optimizing outcomes in ADHD treatment: from clinical targets to novel delivery systems". CNS Spectrums 21 (S1): 45–59. December 2016. doi:10.1017/S1092852916000808. PMID 28044946. https://digitalcommons.wustl.edu/open_access_pubs/5727.
 |