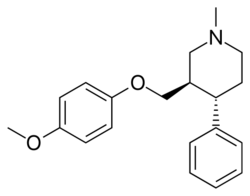
Chemistry:Femoxetine
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 7–27 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C20H25NO2 |
| Molar mass | 311.425 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Femoxetine (INN; tentative brand name Malexil; developmental code name FG-4963) is a drug related to paroxetine that was being developed as an antidepressant by Danish pharmaceutical company Ferrosan in 1975 before acquisition of the company by Novo Nordisk. It acts as a selective serotonin reuptake inhibitor (SSRI). Development was halted to focus attention on paroxetine instead, as femoxetine could not be administered as a daily pill.
Both femoxetine and paroxetine were invented in the 1970s. Jørgen Anders Christensen's name is on the patents[1][2] and Jorgen Buus-Lassen's name is on the pharmacology paper.[3]
After Ferrosan's acquisition, femoxetine died from neglect.[4]
In a separate patent, Ferrosan stated that Femoxetine could be used as an appetite suppressant,[5] using ten times the dosage than for paroxetine, 300 - 400mg daily.
Femoxetine has the same stereochemical properties as Nocaine, another agent with a similar structure claimed to have been synthesized using arecoline as the starting alkaloid.[citation needed]
Analogs
- Addition of the para-fluoro atom results in a different compound that is a hybrid of femoxetine & paroxetine named FG 7080,[6] which has a separate patent.[7] According to the patent tables, incorporation of the fluorine atom potentiated the 5-HT affinity considerably.
- Pfizer made some similar analogs[8] E.g. a Viloxazine type of catechol ether is used, but 4-phenyl instead of based on a morpholine ring.
- NNC-63-0780.[9][10] binds to ORL1 instead of SERT.
- NNC 09-0026
See also
References
- ↑ U.S. Patent 3,912,743
- ↑ U.S. Patent 4,007,196
- ↑ "Comparative studies of a new 5HT-uptake inhibitor and some tricyclic thymoleptics". European Journal of Pharmacology 32 (1): 108–15. May 1975. doi:10.1016/0014-2999(75)90329-5. PMID 1149822.
- ↑ Let them eat Prozac: the unhealthy relationship between the pharmaceutical industry and depression. New York, NY: New York Univ. Press. 2004. pp. 26–27. ISBN 9780814736692. https://archive.org/details/letthemeatprozac00heal. "Jørgen Buus Lassen femoxetine."
- ↑ U.S. Patent 4,442,113
- ↑ "(3S,4R)-4-(4-Fluorophenyl)-3-[(4-methoxyphenoxy)methylpiperidine"]. PubChem. U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/127559.
- ↑ U.S. Patent 4,585,777
- ↑ U.S. Patent 20,070,142,389
- ↑ "Recent advances towards the discovery of ORL-1 receptor agonists and antagonists". Expert Opinion on Therapeutic Patents 15 (4): 357–388 6. 2005. doi:10.1517/13543776.15.4.357.
- ↑ "CID:9862655". PubChem. U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/9862655.
 |
