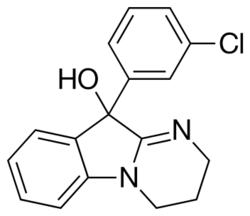
Chemistry:Ciclazindol
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Pharmacokinetic data | |
| Metabolism | Renal[1] |
| Elimination half-life | ~32 hours[1] |
| Excretion | Urine, feces[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C17H15ClN2O |
| Molar mass | 298.77 g·mol−1 |
| 3D model (JSmol) | |
| |
Ciclazindol (WY-23409) is an antidepressant and anorectic[2] drug of the tetracyclic[citation needed] chemical class that was developed in the mid to late 1970s, but was never marketed.[3][4] It acts as a norepinephrine reuptake inhibitor, and to a lesser extent as a dopamine reuptake inhibitor.[3][5] Ciclazindol has no effects on the SERT, 5-HT receptors, mACh receptors, or α-adrenergic receptors, and has only weak affinity for the H1 receptor.[5][6][7] As suggested by its local anesthetic properties,[6] ciclazindol may also inhibit sodium channels. It is known to block potassium channels as well.[8][9]
The dosage in human volunteers is stated to be 25mg daily.[1] However, doses of up to 200mg have also been reported.[2] This is surprising since the dosage of mazindol is only 2-4mg per day.
Ciclazindol is reported to have an IC50 of 1.3nM for the dopamine transporter (cmp 23).[10]
See also
References
- ↑ 1.0 1.1 1.2 1.3 "The pharmacokinetics of ciclazindol (Wy 23409) in human volunteers". British Journal of Clinical Pharmacology 4 (1): 61–65. February 1977. doi:10.1111/j.1365-2125.1977.tb00668.x. PMID 843425.
- ↑ 2.0 2.1 "Ciclazindol: an oral agent with weight reducing properties and hypoglycaemic activity". European Journal of Clinical Pharmacology 25 (1): 41–5. 1983. doi:10.1007/BF00544012. PMID 6352281.
- ↑ 3.0 3.1 "Antidepressant activity and pharmacological interactions of ciclazindol". Psychopharmacology 57 (1): 109–114. April 1978. doi:10.1007/BF00426966. PMID 96461.
- ↑ "A controlled comparative trial of a new antidepressant, ciclazindol". The Journal of International Medical Research 7 (1): 1–6. 1979. doi:10.1177/030006057900700101. PMID 369921.
- ↑ 5.0 5.1 "Influence of ciclazindol on monoamine uptake and CNS function in normal subjects". Psychopharmacology 60 (2): 177–181. January 1979. doi:10.1007/BF00432290. PMID 106428.
- ↑ 6.0 6.1 "Cardiovascular and autonomic actions of ciclazindol and tricyclic antidepressants". Archives Internationales de Pharmacodynamie et de Therapie 240 (1): 116–136. July 1979. PMID 507990.
- ↑ "The effects of mianserine, amitriptyline, ciclazindol and viloxazine on presynaptic alpha-receptors in isolated rat atria [proceedings"]. British Journal of Pharmacology 68 (1): 184P–185P. January 1980. doi:10.1111/j.1476-5381.1980.tb10705.x. PMID 6244029.
- ↑ "The involvement of potassium channels in the action of ciclazindol in rat portal vein". British Journal of Pharmacology 106 (1): 17–24. May 1992. doi:10.1111/j.1476-5381.1992.tb14286.x. PMID 1504725.
- ↑ "Ciclazindol inhibits ATP-sensitive K+ channels and stimulates insulin secretion in CR1-G1 insulin-secreting cells". Molecular Pharmacology 49 (4): 715–720. April 1996. PMID 8609901. http://molpharm.aspetjournals.org/cgi/pmidlookup?view=long&pmid=8609901.
- ↑ "Halogenated mazindol analogs as potential inhibitors of the cocaine binding site at the dopamine transporter". Journal of Medicinal Chemistry 39 (25): 4935–4941. December 1996. doi:10.1021/jm960288w. PMID 8960553.
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| Central |
| ||||||
|---|---|---|---|---|---|---|---|
| Peripheral | |||||||
| |||||||
| DAT (DRIs) |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NET (NRIs) |
| ||||||||||||||
| SERT (SRIs) |
| ||||||||||||||
| VMATs | |||||||||||||||
| Others |
| ||||||||||||||
| Calcium |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium |
| ||||||||||||||||||||||||
| Sodium |
| ||||||||||||||||||||||||
| Chloride |
| ||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||
| Classes | |
|---|---|
| Antidepressants (TCAs and TeCAs) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Others |
|
 | 0.00      (0 votes) (0 votes) |