Chemistry:Bicifadine
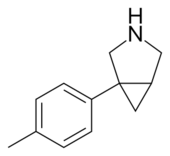 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 1.6 hours |
| Excretion | renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C12H15N |
| Molar mass | 173.259 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Bicifadine (DOV-220,075) is a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI) discovered at American Cyanamid as an analgesic drug candidate, and licensed to DOV Pharmaceutical in 1998 after American Cyanamid was acquired by Wyeth.[1][2][3]
In January 2007, Dov licensed the rights to bicifadine to XTL Biopharmaceuticals after bicifadine failed in a Phase III clinical trial for chronic lower back pain.[4][5][6] XTL ran a PhaseIIb clinical trial for pain caused by diabetic neuropathy, which failed in 2008;[7] XTL terminated the agreement in 2010.[8] In 2010 Dov was acquired by Euthymics Bioscience which intended to continue development of other candidates from Dov's portfolio.[9]
Bicifadine has a non-opioid, non-NSAID mechanism for the treatment of pain, which should have less abuse potential than opioid drugs and less propensity to cause gastric ulcers than NSAID drugs.[10] While the drug is purported to be a serotonin (SERT) and noradrenaline transporter (NET) inhibitor, it also has effects at the dopamine transporter (DAT), effectively making it a broad-spectrum monoamine transporter inhibitor or "triple reuptake inhibitor."[11]
See also
References
- ↑ "Triple reuptake inhibitors: a premise and promise". Psychiatry Investigation 5 (3): 142–7. September 2008. doi:10.4306/pi.2008.5.3.142. PMID 20046357.
- ↑ SEC Filing: Wyeth-DOV Restated License Agreement Page accessed July 15, 2015]
- ↑ "Indiplon". GABA and Sleep: Molecular, Functional and Clinical Aspects. Springer Science & Business Media. 2010. pp. 453––464. ISBN 9783034602266.
- ↑ "Bear Out of Hibernation". BioCentury. 26 July 2010. https://www.biocentury.com/biocentury/finance/2010-07-26/euthymics-bioscience-gets-24m-vcs-revive-cns-assets-dov-pharmafrench?customType=sResults_%2Fsearch%2Fbicifadine&kwh=bicifadine%3C%7C%3Ebicifadine.
- ↑ "Euthymics: Balancing act". BioCentury, The Bernstein Report on Biobusiness. December 5, 2011. p. A13. http://euthymics.com/wp-content/uploads/2011/12/EUTHYMICS_BIOSCIENCE_ECP_BERNSTEIN_REPORT_120511bc1.pdf.
- ↑ "XTL licenses development rights to pain therapy". Fierce Biotech. 7 January 2007. http://www.fiercebiotech.com/story/xtl-licenses-development-rights-to-pain-therapy/2007-01-16.
- ↑ Fierce Biotech. December 9, 2008 Tiny XTL cuts costs, jobs
- ↑ "XTL Form 6-K March, 2013". http://markets.on.nytimes.com/research/stocks/fundamentals/drawFiling.asp?docKey=137-000114420413017126-72672IPVQ4UFBUKR6AA4FI0HPE&docFormat=HTM&formType=6-K.
- ↑ Fierce Biotech July 22, 2010 Euthymics lands $24M to fund antidepressant work
- ↑ "The oral analgesic efficacy of bicifadine hydrochloride in postoperative pain". Journal of Clinical Pharmacology 22 (4): 160–4. April 1982. doi:10.1002/j.1552-4604.1982.tb02157.x. PMID 7096604.
- ↑ "Characterization of the antinociceptive actions of bicifadine in models of acute, persistent, and chronic pain". The Journal of Pharmacology and Experimental Therapeutics 321 (3): 1208–25. June 2007. doi:10.1124/jpet.106.116483. PMID 17325229.
 |

