Chemistry:Dasotraline
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C16H15Cl2N |
| Molar mass | 292.20 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
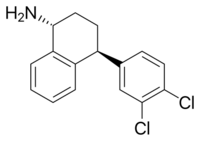
Dasotraline (INN;[1] former developmental code name SEP-225,289) is a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI) that was under development by Sunovion for the treatment of attention-deficit hyperactivity disorder (ADHD) and binge eating disorder (BED).[2][3][4][5] Structurally, dasotraline is a stereoisomer of desmethylsertraline (DMS), which is an active metabolite of the marketed selective serotonin reuptake inhibitor (SSRI) antidepressant sertraline (Zoloft).
Adverse Effects
In phase I clinical trials for attention deficit hyperactivity disorder, test subjects reported the following side effects:[6]
- Loss of appetite
- Dehydration
- Dry mouth
- Nausea
- Insomnia
- Anxiety
- Panic attacks
- Headaches
History
In 2017, the U.S. Food and Drug Administration accepted Sunovion's New Drug Application (NDA) for review for the treatment of ADHD;[7] however, the NDA was ultimately rejected citing the need for additional studies to determine efficacy and tolerability.[8][9][10] In July 2019, Sunovion’s NDA for the treatment of BED was accepted with an expected action date of May 2020.[11] In May 2020, Sunovion halted its drug development program for dasotraline, withdrawing both NDAs for ADHD and BED.[12]
Law
Finland
Dasotraline is completely unscheduled.
See also
References
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN)". WHO Drug Information (WHO) 27 (4). 2013. https://www.who.int/medicines/publications/druginformation/PL_110.pdf. Retrieved 4 November 2014.
- ↑ "Triple uptake inhibitors: therapeutic potential in depression and beyond". Expert Opinion on Investigational Drugs 16 (9): 1365–77. September 2007. doi:10.1517/13543784.16.9.1365. PMID 17714023.
- ↑ "SEP-225289 serotonin and dopamine transporter occupancy: a PET study". Journal of Nuclear Medicine 52 (7): 1150–5. July 2011. doi:10.2967/jnumed.110.084525. PMID 21680689.
- ↑ "[Arthrographic imaging of ganglions of the hand]" (in de). RöFo 153 (2): 143–6. August 1990. doi:10.1055/s-2008-1033352. PMID 2168068.
- ↑ "P.1.c.059 Electrophysiological properties of the triple reuptake inhibitor SEP 225289 on monoaminergic neurons". European Neuropsychopharmacology 19: S285. 2009. doi:10.1016/S0924-977X(09)70419-5.
- ↑ "Dasotraline for the Treatment of Attention-Deficit/Hyperactivity Disorder: A Randomized, Double-Blind, Placebo-Controlled, Proof-of-Concept Trial in Adults". Neuropsychopharmacology 40 (12): 2745–52. November 2015. doi:10.1038/npp.2015.124. PMID 25948101.
- ↑ "Sunovion Announces FDA Acceptance for Review of New Drug Application for Dasotraline for the Treatment of ADHD" (Press release). Marlborough, Massachusetts: Sunovion. Business Wire. November 10, 2017. Archived from the original on 2018-05-01. Retrieved 2018-05-01.
- ↑ "FDA Issues a Complete Response Letter for New Drug Application for Dasotraline for the Treatment of Adhd". Sumitomo Dainippon Pharma Co., Ltd.. August 31, 2018. https://news.sunovion.com/press-release/fda-issues-complete-response-letter-new-drug-application-dasotraline-treatment-adhd.
- ↑ "Pipeline Report: Brand Drugs". Welldyne. February 2018. pp. 1, 4. https://www.welldynerx.com/content/uploads/2018/02/PipelineReport-Brand-Drugs.pdf.
- ↑ Clinical trial number NCT02276209 for "Dasotraline Adult ADHD Study" at ClinicalTrials.gov
- ↑ "Sunovion Announces Acceptance by the US FDA of the New Drug Application for Dasotraline for the Treatment of Adults with Moderate-to-Severe Binge Eating Disorder". Sumitomo Dainippon Pharma Co., Ltd.. July 30, 2019. https://news.sunovion.com/press-release/sunovion-announces-acceptance-us-fda-new-drug-application-dasotraline-treatment.
- ↑ "Sunovion Discontinues Dasotraline Program" (in en). 13 May 2020. https://www.businesswire.com/news/home/20200513005363/en/Sunovion-Discontinues-Dasotraline-Program.
Further reading
- Liming Shao Patent
- Shao L, Wang F, Malcolm SC, Hewitt MC, Bush LR, Ma J, Varney MA, Campbell U, Engel SR, Hardy LW, Koch P, Campbell JE, "Cycloalkylamines as monoamine reuptake inhibitors", US patent application 2007203111, published 2007-08-30, assigned to Sepracor Inc.
- Asymmetry Patent
- Han X, Krishnamurthy D, Senanayake CH, Lu Z-H, "Method of preparing amine stereoisomers", US patent 7129378, published 2005-07-28, assigned to Apsinterm LLC
 |

