Chemistry:Diphenylprolinol
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
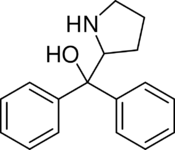
| Formula | C17H19NO |
| Molar mass | 253.345 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Diphenylprolinol (D2PM), or (R/S)-(±)-diphenyl-2-pyrrolidinyl-methanol, is a norepinephrine-dopamine reuptake inhibitor which is used as a designer drug.[1]
Pharmacology
The dextrorotary (R)-(+)-enantiomer is the more pharmacologically active, although a variety of related derivatives have been studied.[2]
Side effects including chest pain (suggestive of possible cardiovascular toxicity) have been seen following recreational use of diphenylprolinol, although it was combined with glaucine in a party pill product, thus making it impossible to say for certain which drug was responsible.[3]
Other uses
Diphenylprolinol can be used to prepare the chiral CBS catalyst, which is used for enantioselective organic synthesis.[4]
See also
- 2-Diphenylmethylpyrrolidine (Desoxy-diphenylprolinol)
- Desoxypipradrol
- Pipradrol
- Prolinol
- Corey-Bakshi-Shibata reduction
References
- ↑ "Detection of the novel recreational drug diphenyl-2-pyrrolidinemethanol (D2PM) sold'legally'in combination with glaucine.". Clinical Toxicology 46 (5): 393. June 2008.; "Abstracts of the XXVIII International Congress of the European Association of Poison Centres and Clinical Toxicologists. May 6-9, 2008. Seville, Spain". Clinical Toxicology 46 (5): 351–421. June 2008. doi:10.1080/15563650802071703. PMID 18568796.
- ↑ US patent 5925666, Jackson PF, Slusher BS, "Pharmaceutical compositions and methods for treating compulsive disorders using pyrrolidine derivatives", issued 20 July 1999, assigned to Eisai Corp of North America.
- ↑ "Cardiovascular toxicity associated with recreational use of diphenylprolinol (diphenyl-2-pyrrolidinemethanol [D2PM)"]. Journal of Medical Toxicology 4 (3): 167–169. September 2008. doi:10.1007/bf03161195. PMID 18821489.
- ↑ "Highly enantioselective borane reduction of ketones catalyzed by chiral oxazaborolidines Mechanism and synthetic implications". J. Am. Chem. Soc. 109 (18): 5551–5553. 1987. doi:10.1021/ja00252a056. ISSN 0002-7863.
 |

