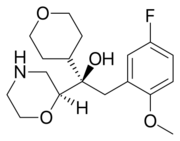
Chemistry:Edivoxetine
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C18H26FNO4 |
| Molar mass | 339.407 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Edivoxetine (INN; code name LY-2216684) is a drug which acts as a selective norepinephrine reuptake inhibitor and was under development by Eli Lilly for attention-deficit disorder (ADD) and as an antidepressant treatment.[1][2] It was in phase III clinical trials in 2012 for major depressive disorder, but failed to get approval.[1][3]
Effectiveness
In a study published in 2010, edivoxetine succeeded to prove superiority over placebo, as measured by Hamilton Depression Rating Scale. However, effectiveness could be observed using the Self-Rated Quick Inventory of Depressive Symptomatology.[4]
In a study published in 2011, using the Montgomery–Åsberg Depression Rating Scale and the Sheehan Disability Scale, edivoxetine showed superiority over placebo, with higher response and remission rates.[5]
In December 2013, Eli Lilly announced that the clinical development of edivoxetine will be stopped due to lack of efficacy compared to SSRI alone in three separate clinical trials.[6]
Side effects
Side effects significantly associated with edivoxetine are headache, nausea, constipation, dry mouth and insomnia.[4]
The above-mentioned studies report increases of the cardiac rhythm, and one also increases of diastolic and systolic blood pressures.[4][5]
See also
References
- ↑ 1.0 1.1 Jun Yan (March 2012). Pipeline for new antidepressants flowing slowly. 47. p. 1b–29. doi:10.1176/pn.47.5.psychnews_47_5_1-b. http://dsm.psychiatryonline.org/newsarticle.aspx?articleid=1032188. Retrieved 2012-04-27.
- ↑ "Statement on a nonproprietary name adopted by the USAN council - Edivoxetine" (PDF) (Press release). American Medical Association. 2012. Retrieved 2012-04-12.
- ↑ Chancellor D (November 2011). "The depression market". Nature Reviews. Drug Discovery 10 (11): 809–10. doi:10.1038/nrd3585. PMID 22037032.
- ↑ 4.0 4.1 4.2 "A study of the effects of LY2216684, a selective norepinephrine reuptake inhibitor, in the treatment of major depression". Journal of Psychiatric Research 44 (6): 356–363. April 2010. doi:10.1016/j.jpsychires.2009.09.013. PMID 19909980.
- ↑ 5.0 5.1 "A randomized, double-blind study comparing LY2216684 and placebo in the treatment of major depressive disorder". Journal of Psychiatric Research 45 (6): 748–755. June 2011. doi:10.1016/j.jpsychires.2011.03.014. PMID 21511276.
- ↑ "Lilly Announces Edivoxetine Did Not Meet Primary Endpoint of Phase III Clinical Studies as Add-On Therapy for Major Depressive Disorder | Eli Lilly and Company". https://investor.lilly.com/releasedetail.cfm?ReleaseID=811751.
 |
