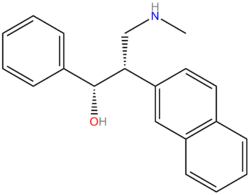
Chemistry:PRC200
 | |
| Identifiers | |
|---|---|
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C20H21NO |
| Molar mass | 291.386 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
PRC200-SS is an arylalkanolamine TRI being developed by the Mayo Clinic.[1][2] Sympathomimetic PRC200-SS is the PRC050 eutomer,[3] whereas PRC201 is the distomer. These compounds are preceded by venlafaxine, which Wyeth claims is the first SNRI.[4] Venlafaxine was originally developed as an "opioid" although original screening returned negative results.[4] The authors were not satisfied just to drop venlafaxine from development and continued with their study of the compounds biological activity data. Herein, they discovered that venlafaxine exerts its biological actions via interaction with the monoamine receptors. In particular, the actions of the drug on increasing the amount of 5-HT and NE were documented,[4] although with "potentiated" analogs such as the pm-dichlorophenyl ring substituted derivative, it might be expected to behave as a SNDRI also (but no data was available to support this inference). Venlafaxine itself has been said to behave as a SNDRI at very high doses. This would be more likely to be the case in drug naïve subjects than in users that have already built up significant tolerance.
Silicon containing analog of venlafaxine was prepared and demonstrated to be an active SNRI.
Chirality
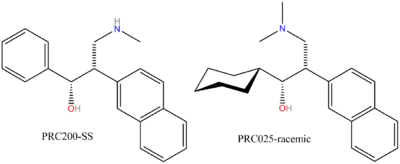
For venlafaxine there is only one chiral centre, although for the PRC compounds, there is a diastereoisomeric pair of racemers to consider.
The exact choice of conditions (e.g. temperature and choice of solvent, etc.) can be altered to try to increase the dia/stereo-selectivity.
PRC050 is racemic SS/RR, PRC025 is racemic SR/RS,[3] venlafaxine is racemic;
PRC050 was further resolved into its constituent enantiomers, PRC200 (SS) and PRC201 (RR), respectively.[2]
| Compound | hNET Kd (Ki) | hSERT Kd (Ki) | hDAT Kd (Ki) |
|---|---|---|---|
| Venlafaxine | 1060 (210) | 9.0 (39) | 9,300 (5,300) |
| PRC025 | 19 (10) | 6.0 (6.0) | 100 (53) |
| PRC050 | 0.40 (1.2) | 6.0 (12) | 120 (43) |
| PRC200-SS | 0.63 (1.5) | 2.3 (2.1) | 18 (61) |
| PRC201 | 42 (?) | 210 (?) | 200 (?) |
As can be seen in the above table, a high eudysmic ratio exists for PRC050 with activity residing in the SS enantiomer (PRC200).
Type of amine
In contrast to venlafaxine and PRC025, PRC050 is a secondary amine (and not a tertiary amine).
This is like comparing imipramine with desipramine, or amitriptyline with nortriptyline.
N-demethylation has the effect of boosting noradrenergic activity, but does not increase binding to the dopamine active transporter.
Transporter selectivity
PRC025 has the in vitro MAT order of potency S>N>D whereas for PRC050 the affinity for the transporters is N>S>D.
Currently the thinking is that PRC050 should be preferred to PRC025 on the basis that it is the more potent of the two compounds.
Liang and Richelson actually believe that a nomifensine type analog with an order of potency N>D>S would be optimal.
Their reasoning for N>D is that this would limit abuse liability, and D>S is that this would lessen the risk of serotonin syndrome.
Behavioral studies on rodents
Only PRC200 was considered further in behavioral studies.[2]
PRC200-SS is active in the FST and the TST at a dose of 5 mg/kg, results are similar to imipramine.
PRC200-SS is much more potent than imipramine: 1 mg/kg of PRC200-SS is ≈ equivalent to 15 mg/kg imipramine.
PRC200-SS does not cause LMA and is not self-administered by the rodents, meaning it is unlikely to be reinforcing.
Increasing the dose to 10 mg/kg does not enhance the activity of PRC200-SS further, indicating that 5 mg/kg is the opimal dosage.
Toxicity
Trials of PRC200-SS in cynomolgus monkeys showed dose proportional kidney toxicity, with signs that the compound was damaging to the distal tubule and collecting duct.[5] This adverse result makes it unlikely that PRC200-SS will be developed for clinical use in humans, though development of related compounds may well continue.
Microdialysis
Concentrations of NE, 5-HT, DA, DOPAC, HVA, and 5-HIAA, were measured in the mPFC and NAc, respectively.[2]
At 10 mg/kg NE concentrations were increased by c.f. ~700% respectively in the mPFC.
This is in contrast to the core of the NAc where concentrations of NE were not elevated at all.
The authors rationalized that this is because all the NET dense fibres/tissue lies in the NAc shell and not in the core (?)
In the mPFC concentrations of DA were not elevated at all. Authors claim that this is because of the low density of DAT tissue in the mPFC.
However, in the core of the NAc, DA concentrations were elevated by c.f. ~160% upon administration of 10 mg/kg of PRC200-SS.
This is consistent with the drop in cytoplasmic concentrations of HVA and DOPAC that were also measured in this brain region at this dose.
An elevation in the concentration of 5-HT and reduction in the concentration of 5-HIAA were measured, particularly in the mPFC;
however the % change from baseline was less than expected on the basis of the in vitro measurements that were recorded.
Authors resorted to the theory that is known about 5-HT1A (and related) autoreceptors to try to help account for this observation.
SAR
In the initial assessment of these compounds, analogs displaying an increased role at the DAT relative to the other 2 transporters was sought.
| R | Ar | N | SERT | 5HT | NET | NE | DAT | DA |
| Ph | Ph | H2 | 3,050 | 2,730 | 12,500 | 5,780 | 24K | 6,600 |
| c-C6H11 | p-anisyl | H2 | 76 | 164 | 1,540 | 330 | 4,310 | 1,460 |
| mesityl | Ph | H2 | 118 | 643 | 22,500 | 8,600 | 340 | 5,310 |
| t-Bu | Ph | H2 | 1,100 | 14K | 47,900 | 3,800 | 7,260 | 4,570 |
| t-Bu | 2-naph | H2 | 6.1 | 14 | 55 | 44 | 27K | 120 |
| Ph | 2-naph | H2 | 6.2 | 27 | 21 | 7.7 | 140 | 6.2 |
| Ph | Ph | Me2 | 48 | 210 | 2,250 | 990 | 12,000 | 1,550 |
| c-C6H11 | p-anisyl | Me2 | 30 | 30 | 1,800 | 220 | 3,500 | 640 |
| mesityl | Ph | Me2 | 8.5 | 57 | 35,800 | 8,500 | 42K | 4,880 |
| t-Bu | Ph | Me2 | 40 | 60 | 3,430 | 830 | 21,400 | 1,200 |
| t-Bu | 2-naph | Me2 | 1.2 | 2.6 | 29.9 | 10 | 340 | 60 |
| Ph | 2-naph | Me2 | 5.59 | 4.1 | 44 | 15 | 70 | 12 |
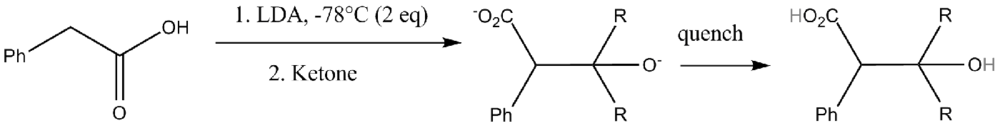
Syntheses
Nitrile-aldol conditions ensures desired anti-addition product.[6][7][8]

Patents
U.S. Patent 6,069,177 U.S. Patent 6,700,018 U.S. Patent 6,914,080
Latest developments
Richelsen, et al. have continued modifying the basic structures.
Original document: US2013059864 (A1) ― 2013-03-07 Original document: US2012220665 (A1) ― 2012-08-30
For example, if read this citation it can be read what is happening.[10][11]
Get the basic PRC200-SS structure, then undergone further chemical permutations.
+ SOCl2 → MCJ001Cl-SS
+ DAST → MCJ001F-SS
+ HN3, PPh3 (DIAD) → MCJ001N3-SS
References
- ↑ Y. Liang, E. Richelson. Triple Reuptake Inhibitors: Next-Generation Antidepressants. Primary Psychiatry. 2008;15(4):50-56.
- ↑ 2.0 2.1 2.2 2.3 Liang, Y.; Shaw, A.; Boules, M.; Briody, S.; Robinson, J.; Oliveros, A.; Blazar, E.; Williams, K. et al. (2008). "Antidepressant-like pharmacological profile of a novel triple reuptake inhibitor, (1S,2S)-3-(methylamino)-2-(naphthalen-2-yl)-1-phenylpropan-1-ol (PRC200-SS)". The Journal of Pharmacology and Experimental Therapeutics 327 (2): 573–583. doi:10.1124/jpet.108.143610. PMID 18689611.
- ↑ 3.0 3.1 Shaw, A.; Boules, M.; Zhang, Y.; Williams, K.; Robinson, J.; Carlier, P.; Richelson, E. (2007). "Antidepressant-like effects of novel triple reuptake inhibitors, PRC025 and PRC050". European Journal of Pharmacology 555 (1): 30–36. doi:10.1016/j.ejphar.2006.10.004. PMID 17109850.
- ↑ 4.0 4.1 4.2 Carlier, P. R.; Lo, M. M.; Lo, P. C.; Richelson, E.; Tatsumi, M.; Reynolds, I. J.; Sharma, T. A. (1998). "Synthesis of a potent wide-spectrum serotonin-, norepinephrine-, dopamine-reuptake inhibitor (SNDRI) and a species-selective dopamine-reuptake inhibitor based on the gamma-amino alcohol functional group". Bioorganic & Medicinal Chemistry Letters 8 (5): 487–492. doi:10.1016/S0960-894X(98)00062-6. PMID 9871604.
- ↑ "Assessment of Biomarkers of Drug-induced Kidney Injury in Cynomolgus Monkeys Treated with a Triple Re-uptake Inhibitor". Toxicological Sciences 120 (2): 269–83. January 2011. doi:10.1093/toxsci/kfr013. PMID 21258088.
- ↑ Carlier, P. R.; Lo, K. M.; Lo, M. M. C.; Williams, I. D. (1995). "Anti-Selective Aldol Reaction of Benzylic Nitriles and Synthesis of .gamma.-Amino Alcohols". The Journal of Organic Chemistry 60 (23): 7511. doi:10.1021/jo00128a025.
- ↑ Carlier, P. R.; Lo, K. M. (1994). "2,3-Anti Selective Aldol Reaction of Phenylacetonitrile". The Journal of Organic Chemistry 59 (15): 4053. doi:10.1021/jo00094a011.
- ↑ Carlier, P. R.; Lo, K. M.; Lo, M. M. -C.; Lo, P. C. -K.; Lo, C. W. -S. (1997). "Synthetic Optimization and Structural Limitations of the Nitrile Aldol Reaction". The Journal of Organic Chemistry 62 (18): 6316. doi:10.1021/jo9702148.
- ↑ Vu, A.; Cohn, S.; Terefenko, E.; Moore, W.; Zhang, P.; Mahaney, P.; Trybulski, E.; Goljer, I. et al. (2009). "3-(Arylamino)-3-phenylpropan-2-olamines as a new series of dual norepinephrine and serotonin reuptake inhibitors". Bioorganic & Medicinal Chemistry Letters 19 (9): 2464–2467. doi:10.1016/j.bmcl.2009.03.054. PMID 19329313. Mahaney, P.; Gavrin, L.; Trybulski, E.; Stack, G.; Vu, T.; Cohn, S.; Ye, F.; Belardi, J. et al. (2008). "Structure-activity relationships of the cycloalkanol ethylamine scaffold: discovery of selective norepinephrine reuptake inhibitors". Journal of Medicinal Chemistry 51 (13): 4038–4049. doi:10.1021/jm8002262. PMID 18557608.
- ↑ Valenta, P.; Carroll, P. J.; Walsh, P. J. (2010). "Stereoselective Synthesis of β-Hydroxy Enamines, Aminocyclopropanes, and 1,3-Amino Alcohols via Asymmetric Catalysis". Journal of the American Chemical Society 132 (40): 14179. doi:10.1021/ja105435y. PMID 20853837.
- ↑ http://repository.upenn.edu/cgi/viewcontent.cgi?article=1348&context=edissertations
