Chemistry:Machaeriol A
From HandWiki
Short description: Chemical compound
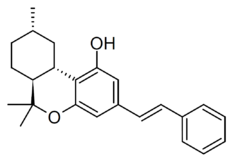 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C24H28O2 |
| Molar mass | 348.486 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Machaeriol A is one of a number of phytocannabinoids with a hexahydrocannabinol backbone, found in plants from the Machaerium family such as Machaerium multiflorum. While they are related in structure to tetrahydrocannabinols such as those from cannabis, the machaeriol compounds have opposite trans stereochemistry from THC and have no affinity for the psychoactive CB1 receptor. However, some derivatives are active at CB2, and they have also been found to have antibacterial, antifungal and antiparasitic actions, and have been investigated as lead compounds for the development of potential anti-cancer drugs.[1][2][3][4][5][6][7][8][9][10]
See also
References
- ↑ "Antimalarial (+)-trans-hexahydrodibenzopyran derivatives from Machaerium multiflorum". Journal of Natural Products 64 (10): 1322–1325. October 2001. doi:10.1021/np0102861. PMID 11678659.
- ↑ "Antimicrobial and antiparasitic (+)-trans-hexahydrodibenzopyrans and analogues from Machaerium multiflorum". Journal of Natural Products 66 (6): 804–809. June 2003. doi:10.1021/np030045o. PMID 12828466.
- ↑ "Total Synthesis of (+)‐Machaeriols B and C and of Their Enantiomers with a Cannabinoid Structure.". Helvetica Chimica Acta 92 (7): 1404–1412. July 2009. doi:10.1002/hlca.200900014.
- ↑ "Short and Divergent Total Synthesis of (+)-Machaeriol B, (+)-Machaeriol D, (+)-Δ(8)-THC, and Analogues". Angewandte Chemie 54 (29): 8547–8550. July 2015. doi:10.1002/anie.201502595. PMID 26079816.
- ↑ "An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol". Frontiers in Pharmacology 8: 422. 2017. doi:10.3389/fphar.2017.00422. PMID 28701957.
- ↑ "Synthetic pathways to tetrahydrocannabinol (THC): an overview". Organic & Biomolecular Chemistry 18 (17): 3203–3215. May 2020. doi:10.1039/d0ob00464b. PMID 32259175.
- ↑ "Antimicrobial Constituents from Machaerium Pers.: Inhibitory Activities and Synergism of Machaeriols and Machaeridiols against Methicillin-Resistant Staphylococcus aureus, Vancomycin-Resistant Enterococcus faecium, and Permeabilized Gram-Negative Pathogens". Molecules 25 (24): 6000. December 2020. doi:10.3390/molecules25246000. PMID 33352963.
- ↑ "Novel Machaeriol Analogues as Modulators of Cannabinoid Receptors: Structure-Activity Relationships of (+)-Hexahydrocannabinoids and Their Isoform Selectivities". ACS Omega 6 (31): 20408–20421. August 2021. doi:10.1021/acsomega.1c02413. PMID 34395989.
- ↑ "Synthesis and Inhibitory Activity of Machaeridiol-Based Novel Anti-MRSA and Anti-VRE Compounds and Their Profiling for Cancer-Related Signaling Pathways". Molecules 27 (19): 6604. October 2022. doi:10.3390/molecules27196604. PMID 36235141.
- ↑ "Cannabinoid and Opioid Receptor Affinity and Modulation of Cancer-Related Signaling Pathways of Machaeriols and Machaeridiols from Machaerium Pers". Molecules 28 (10): 4162. May 2023. doi:10.3390/molecules28104162. PMID 37241903.
- ↑ "Machaeriol B". PubChem. U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/1038405.
- ↑ "Machaeriol C". PubChem. U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/9998869.
 |
