Chemistry:QUCHIC
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
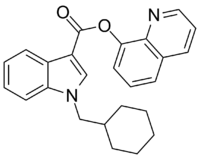
| Formula | C25H24N2O2 |
| Molar mass | 384.479 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
QUCHIC (BB-22, SGT-32 or 1-(cyclohexylmethyl)-1H-indole-3-carboxylic acid 8-quinolinyl ester) is a designer drug offered by online vendors as a cannabimimetic agent, and was first detected being sold in synthetic cannabis products in Japan in early 2013,[1] and subsequently also in New Zealand.[2] The structure of QUCHIC appears to use an understanding of structure-activity relationships within the indole class of cannabimimetics, although its design origins are unclear. QUCHIC, along with QUPIC, represents a structurally unique synthetic cannabinoid chemotype since it contains an ester linker at the indole 3-position rather than the precedented ketone of JWH-018 and its analogues, or the amide of SDB-001 and its analogues.
Pharmacology
BB-22 acts as a full agonist with a binding affinity of 0.217nM at CB1 and 0.338nM at CB2 cannabinoid receptors.[3]
See also
References
- ↑ "Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative α-PVT and an opioid receptor agonist AH-7921 identified in illegal products". Forensic Toxicology 31 (2): 223–240. 2013. doi:10.1007/s11419-013-0182-9.
- ↑ Dunne bans further two substances found in K2. Press Release: New Zealand Government. Tuesday, 30 April 2013
- ↑ "Pharmacological evaluation of synthetic cannabinoids identified as constituents of spice". Forensic Toxicology 34 (2): 329–343. 1 July 2016. doi:10.1007/s11419-016-0320-2. PMID 27429655.
 |

