Chemistry:ADBICA
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID |
|
| ChemSpider |
|
| UNII |
|
| Chemical and physical data | |
| Formula | C20H29N3O2 |
| Molar mass | 343.471 g·mol−1 |
| 3D model (JSmol) |
|
| |
| |
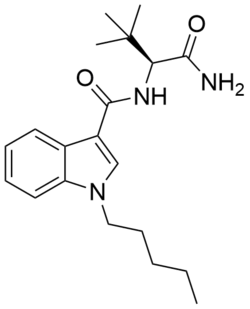
ADBICA (also known as ADB-PICA) is a designer drug identified in synthetic cannabis blends in Japan in 2013.[1] ADBICA had not previously been reported in the scientific literature prior to its sale as a component of synthetic cannabis blends. ADBICA features a carboxamide group at the 3-indole position, like SDB-001 and STS-135. The stereochemistry of the tert-butyl side-chain in the product is unresolved, though in a large series of indazole derivatives structurally similar to ADBICA that are disclosed in Pfizer patent WO 2009/106980, activity resides exclusively in the (S) enantiomers.[2] ADBICA is a potent agonist of the CB1 receptor and CB2 receptor with an EC50 value of 0.69 nM and 1.8 nM respectively.[3]
Legal Status
As of October 2015 ADBICA is a controlled substance in China.[4]
See also
- 5F-AB-PINACA
- 5F-ADB
- 5F-ADBICA
- 5F-AMB
- 5F-APINACA
- AB-FUBINACA
- AB-CHFUPYCA
- AB-CHMINACA
- AB-PINACA
- ADB-CHMINACA
- ADB-FUBINACA
- ADB-PINACA
- ADB-P7AICA
- APICA
- APINACA
- MDMB-CHMICA
- PF-03550096
- STS-135
- PX-3
References
- ↑ "Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative α-PVT and an opioid receptor agonist AH-7921 identified in illegal products". Forensic Toxicology 31 (2): 223–240. 2013. doi:10.1007/s11419-013-0182-9.
- ↑ Buchler IP, et al, "Indazole Derivatives", WO patent 2009/106980, assigned to Pfizer, Inc.
- ↑ "Pharmacology of Indole and Indazole Synthetic Cannabinoid Designer Drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA". ACS Chemical Neuroscience 6 (9): 1546–59. September 2015. doi:10.1021/acschemneuro.5b00112. PMID 26134475.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in zh). China Food and Drug Administration. 27 September 2015. http://www.sfda.gov.cn/WS01/CL0056/130753.html.
 |

