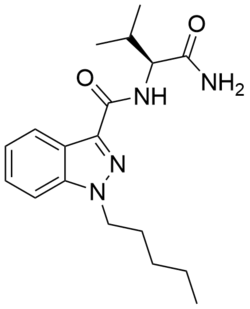
Chemistry:AB-PINACA
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C18H26N4O2 |
| Molar mass | 330.432 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
AB-PINACA is a compound that was first identified as a component of synthetic cannabis products in Japan in 2012.[1]
It was originally developed by Pfizer in 2009 as an analgesic medication.[2][3]
AB-PINACA acts as a potent agonist for the CB1 receptor (Ki = 2.87 nM, EC50 = 1.2 nM) and CB2 receptor (Ki = 0.88 nM, EC50 = 2.5 nM) and fully substitutes for Δ9-THC in rat discrimination studies, while being 1.5x more potent.[4][5]
There have been a number of reported cases of deaths and hospitalizations in relation to this synthetic cannabinoid.[6][7]
Legal status
Germany
AB-PINACA is an Anlage II controlled substance in Germany as of November 2014.[8]
Singapore
It is listed in the Fifth Schedule of the Misuse of Drugs Act and so is illegal in Singapore, as of May 2015.[9]
United States
It is a Schedule I controlled substance in the United States .[10]
China
It is a controlled substance in China as of October 2015.[11]
France
It is a controlled substance in France as of March 2017.[12]
See also
- 5F-AB-PINACA
- 5F-ADB
- 5F-AMB
- 5F-APINACA
- 5F-CUMYL-PINACA
- AB-CHFUPYCA
- AB-FUBINACA
- AB-PICA
- ADB-CHMINACA
- ADB-FUBINACA
- ADB-PINACA
- ADBICA
- APICA
- APINACA
- MDMB-CHMICA
- PX-3
References
- ↑ "New cannabimimetic indazole derivatives, N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide (AB-PINACA) and N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide (AB-FUBINACA) identified as designer drugs in illegal products". Forensic Toxicology 31: 93–100. 2012. doi:10.1007/s11419-012-0171-4.
- ↑ "AB-PINACA". Cayman Chemical. https://www.caymanchem.com/app/template/Product.vm/catalog/14038.
- ↑ Buchler IP, Hayes MJ, Hegde SG, Hockerman SL, Jones DE, Kortum SW, Rico JG, Tenbrink RE, Wu KK, "Indazole derivatives", WO patent 2009106980A, published 3 September 2009, assigned to Pfizer Inc.
- ↑ "Pharmacology of Indole and Indazole Synthetic Cannabinoid Designer Drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA". ACS Chemical Neuroscience 6 (9): 1546–1559. September 2015. doi:10.1021/acschemneuro.5b00112. PMID 26134475.
- ↑ "AB-CHMINACA, AB-PINACA, and FUBIMINA: Affinity and Potency of Novel Synthetic Cannabinoids in Producing Δ9-Tetrahydrocannabinol-Like Effects in Mice". The Journal of Pharmacology and Experimental Therapeutics 354 (3): 328–339. September 2015. doi:10.1124/jpet.115.225326. PMID 26105953.
- ↑ "Synthetic Cannabinoid-Related Illnesses and Deaths". The New England Journal of Medicine 373 (2): 103–107. July 2015. doi:10.1056/NEJMp1505328. PMID 26154784.
- ↑ "Unintentional Pediatric Exposure to a Synthetic Cannabinoid (AB-PINACA) Resulting in Coma and Intubation". Annals of Emergency Medicine 66 (3): 343–344. September 2015. doi:10.1016/j.annemergmed.2015.05.021. PMID 26304261.
- ↑ "Gesetz über den Verkehr mit Betäubungsmitteln (Betäubungsmittelgesetz - BtMG) Anlage II (zu § 1 Abs. 1) (verkehrsfähige, aber nicht verschreibungsfähige Betäubungsmittel)". http://www.gesetze-im-internet.de/btmg_1981/anlage_ii.html.
- ↑ "Misuse of Drugs Act". Central Narcotics Bureau (CNB). 30 April 2015. http://www.cnb.gov.sg/Libraries/CNB_Newsroom_Files/CNB_NR_-_30_Apr_2015.sflb.ashx.
- ↑ "Schedules of controlled substances: Temporary placement of three synthetic cannabinoids into schedule I. Final order". Drug Enforcement Administration, U.S. Department of Justice. 30 January 2015. http://www.gpo.gov/fdsys/pkg/FR-2015-01-30/pdf/2015-01776.pdf.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in zh). China Food and Drug Administration. 27 September 2015. http://www.sfda.gov.cn/WS01/CL0056/130753.html.
- ↑ "Arrêté du 31 mars 2017 modifiant l'arrêté du 22 février 1990 fixant la liste des substances classées comme stupéfiants" (in fr). Legifrance. 6 April 2017. https://www.legifrance.gouv.fr/eli/arrete/2017/3/31/AFSP1710288A/jo/texte.
 |

