Chemistry:Cis-THC
From HandWiki
Short description: Cis enantiomer of tetrahydrocannabidiol
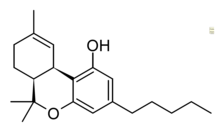 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C21H30O2 |
| Molar mass | 314.469 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
cis-Delta-9-Tetrahydrocannabinol ((-)-cis-Δ9-THC) is an isomer of tetrahydrocannabinol found in the Cannabis plant but in lower quantities than the more well-known trans isomer. It has similar psychoactive effects to trans-Δ9-THC in tests on mice, but with only around 1/5th the potency. The equivalent Δ8 isomer is also known as a synthetic compound, but has not been isolated from Cannabis plant material. All four cis/trans isomers are known, though only the (6aR,10aR) and (6aS,10aR) enantiomers are psychoactive, while the others retain activity at targets such as GPR18 and GPR55.[1][2][3][4][5][6][7][8]
See also
- Abeo-HHC acetate
- Abn-CBD
- CBD-DMH
- Exo-THC
- Iso-THC
- HU-211
- Perrottetinene
- Machaeriol A
References
- ↑ "Hashish. Importance of the phenolic hydroxyl group in tetrahydrocannabinols". Journal of Medicinal Chemistry 18 (2): 213–215. February 1975. doi:10.1021/jm00236a025. PMID 1120991.
- ↑ "Δ1-3, 4-cis-tetrahydrocannabinol in Cannabis sativa.". Phytochemistry 16 (7): 1088–1089. January 1977. doi:10.1016/S0031-9422(00)86745-X. Bibcode: 1977PChem..16.1088S.
- ↑ "Synthetic tetrahydrocannabinol". Journal of Forensic Sciences 28 (3): 762–772. July 1983. doi:10.1520/JFS11571J. PMID 6311937.
- ↑ "High pressure Diels-Alder approach to hydroxy-substituted 6a-cyano-tetrahydro-6H-benzo[c]chromen-6-ones: a route to delta(6)-cis-cannabidiol". The Journal of Organic Chemistry 74 (11): 4311–4317. June 2009. doi:10.1021/jo9005365. PMID 19402693.
- ↑ "High-pressure access to the Δ9-cis- and Δ9-trans-tetrahydrocannabinols family". The Journal of Organic Chemistry 76 (13): 5392–5403. July 2011. doi:10.1021/jo200796b. PMID 21563759.
- ↑ "Stereodivergent total synthesis of Δ9-tetrahydrocannabinols". Angewandte Chemie 53 (50): 13898–13901. December 2014. doi:10.1002/anie.201408380. PMID 25303495.
- ↑ "Δ9-cis-Tetrahydrocannabinol: Natural Occurrence, Chirality, and Pharmacology". Journal of Natural Products 84 (9): 2502–2510. September 2021. doi:10.1021/acs.jnatprod.1c00513. PMID 34304557. https://boris.unibe.ch/161548/.
- ↑ "Brønsted Acid Catalyzed Asymmetric Synthesis of cis-Tetrahydrocannabinoids". Angewandte Chemie 62 (24): e202302475. April 2023. doi:10.1002/anie.202302475. PMID 37057742.
 |


