Chemistry:EAM-2201
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
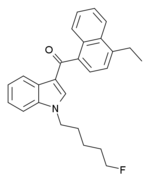
| Formula | C26H26FNO |
| Molar mass | 387.498 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
EAM-2201 (4'-ethyl-AM-2201, 5"-fluoro-JWH-210, SGT-14) is a drug that presumably acts as a potent agonist for the cannabinoid receptors.[1] It had never previously been reported in the scientific or patent literature, and was first identified by laboratories in Japan in July 2012 as an ingredient in synthetic cannabis smoking blends[2] Like the closely related MAM-2201 which had been first reported around a year earlier, EAM-2201 thus appears to be another novel compound invented by designer drug suppliers specifically for recreational use. Structurally, EAM-2201 is a hybrid of two known cannabinoid compounds JWH-210 and AM-2201, both of which had previously been used as active ingredients in synthetic cannabis blends before being banned in many countries.
Pharmacology
EAM-2201 acts as a full agonist with a binding affinity of 0.380 nM at CB1 and 0.371 nM at CB2 cannabinoid receptors.[3]
Legal status
In the United States, all CB1 receptor agonists of the 3-(1-naphthoyl)indole class such as EAM-2201 are Schedule I Controlled Substances.[4]
EAM-2201 was banned in New Zealand as a temporary class drug from 6 December 2012, after reports of addiction and psychosis associated with use of products containing EAM-2201 as an active ingredient, however this has been protested by some users who claim to have found medical benefits in the treatment of conditions such as phantom limb pain, since medicinal marijuana was not available in New Zealand and synthetic cannabis products were used as a legal alternative.[5][6]
EAM-2201 is an Anlage II controlled drug in Germany.
As of October 2015 EAM-2201 is a controlled substance in China.[7]
Detection
A forensic standard of EAM-2201 is available and commonly used in mass spectrometry.[8]
See also
References
- ↑ "In vitro metabolism of a novel synthetic cannabinoid, EAM-2201, in human liver microsomes and human recombinant cytochrome P450s". Journal of Pharmaceutical and Biomedical Analysis 119 (5): 50–8. February 2016. doi:10.1016/j.jpba.2015.11.023. PMID 26641707.
- ↑ "URB-754: a new class of designer drug and 12 synthetic cannabinoids detected in illegal products". Forensic Science International 227 (1–3): 21–32. April 2013. doi:10.1016/j.forsciint.2012.08.047. PMID 23063179. http://www.fsijournal.org/article/S0379-0738%2812%2900434-3/abstract.
- ↑ "Pharmacological evaluation of synthetic cannabinoids identified as constituents of spice". Forensic Toxicology 34 (2): 329–343. 1 July 2016. doi:10.1007/s11419-016-0320-2. PMID 27429655.
- ↑ : Schedules of controlled substances
- ↑ McNeilly, Hamish (1 October 2012). "I was possessed by a demon, says ex-legal high user". New Zealand Herald. http://www.nzherald.co.nz/nz/news/article.cfm?c_id=1&objectid=10837617.
- ↑ "Amputee: K2 'takes away my pain'". Bay of Plenty Times. New Zealand Herald. 28 November 2012. http://www.nzherald.co.nz/nz/news/article.cfm?c_id=1&objectid=10850580.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. http://www.sfda.gov.cn/WS01/CL0056/130753.html.
- ↑ "EAM-2201". Cayman Chemical. https://www.caymanchem.com/app/template/Product.vm/catalog/ISO00127.
 |

