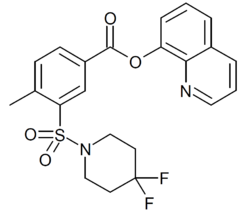
Chemistry:2F-QMPSB
From HandWiki
Short description: Chemical compound
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C22H20F2N2O4S |
| Molar mass | 446.47 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
2F-QMPSB (SGT-13) is an arylsulfonamide-based synthetic cannabinoid that is a fluorinated derivative of QMPSB and has been sold as a designer drug.[1] Its identification was first reported by a forensic laboratory in Italy in January 2019,[2] and it was made illegal in Latvia shortly afterwards.[3][4] Fluorination of the tail group is a common strategy to increase potency at cannabinoid receptors which is seen in many related series of compounds.[5][6]
See also
References
- ↑ "Identification and Analytical Characterization of a Novel Synthetic Cannabinoid-Type Substance in Herbal Material in Europe.". Molecules 26 (4): 793. 2021. doi:10.3390/molecules26040793. PMID 33546439.
- ↑ "NPS2019". Belgian Early Warning System on Drugs (BEWSD). https://ewsd.wiv-isp.be/Lists/NPS20191/Allitemsg.aspx.[yes|permanent dead link|dead link}}]
- ↑ Centre for Disease Prevention and Control (17 January 2019). "Par aizlieguma noteikšanu vielai 2F-QMPSB un tās saturošiem izstrādājumiem" (in Latvian). Latvian Journal: Legal Acts of the Republic of Latvia. https://likumi.lv/ta/id/304366.
- ↑ "Уважаемые эксперты" (in Russian). Aipsin Drugs. https://aipsin.com/files/6/68/.
- ↑ "Effects of bioisosteric fluorine in synthetic cannabinoid designer drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135". ACS Chemical Neuroscience 6 (8): 1445–58. August 2015. doi:10.1021/acschemneuro.5b00107. PMID 25921407. https://zenodo.org/record/47750.
- ↑ "Structure-activity relationships of 2,4-diphenyl-1H-imidazole analogs as CB2 receptor agonists for the treatment of chronic pain". Bioorganic & Medicinal Chemistry Letters 21 (1): 182–5. January 2011. doi:10.1016/j.bmcl.2010.11.044. PMID 21115245.
 |

