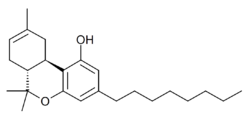
Chemistry:JWH-138
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C24H36O2 |
| Molar mass | 356.550 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
JWH-138 (THC-Octyl, Δ8-THC-C8) is a synthetic cannabinoid first synthesised by John W. Huffman, with a Ki of 8.5nM at the CB1 cannabinoid receptor.[1] THC-Octyl and its hydrogenated analog HHC-Octyl was synthesized and studied by Roger Adams as early as 1942.[2]
Isomers
The Δ3/Δ6a(10a) isomer was synthesised in 1941, but was found to be slightly less active than Δ3-THC itself.[3] The alternate isomer Δ9-THC-C8 has also been synthesised,[4] and both the Δ8 and Δ9 isomers are included within the definition of an "intoxicating cannabinoid" in Colorado under the name tetrahydrocannabioctyl,[5] but it is unclear if it has been identified as a natural product. Tetrahydrocannabioctyl is sometimes referred to as THC-Octyl or THCO, which may cause confusion with THC-O-acetate which is commonly known as THC-O.
See also
References
- ↑ "Manipulation of the tetrahydrocannabinol side chain delineates agonists, partial agonists, and antagonists". The Journal of Pharmacology and Experimental Therapeutics 290 (3): 1065–1079. September 1999. PMID 10454479.
- ↑ https://pubs.acs.org/doi/abs/10.1021/ja01255a061
- ↑ "Tetrahydrocannabinol Homologs with Marihuana Activity. IX.". Journal of the American Chemical Society 63 (7): 1971–1973. July 1941. doi:10.1021/ja01852a052.
- ↑ Abdur-Rashid, Kamaluddin; Wenli Jia & Kareem Abdur-Rashid, "Catalytic cannabinoid processes and precursors", WO patent application 2020232545, published 2020-11-26, assigned to Kare Chemical Technologies Inc..
- ↑ Senate Bill 23-271, General Assembly, State of Colorado
 |



