Chemistry:Leu-enkephalin
From HandWiki
Short description: Neurotransmitter
| |
| Names | |
|---|---|
| IUPAC name
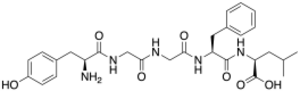
(2R)-2-[[(2R)-2-[[2-[[2-[[(2R)-2-amino-3-
| |
| Other names
[Leu]enkephalin; [Leu5]enkephalin;
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C28H37N5O7 | |
| Molar mass | 555.62 g/mol[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Leu-enkephalin is an endogenous opioid peptide neurotransmitter with the amino acid sequence Tyr-Gly-Gly-Phe-Leu that is found naturally in the brains of many animals, including humans.[2][3] It is one of the two forms of enkephalin; the other is met-enkephalin.[2] The tyrosine residue at position 1 is thought to be analogous to the 3-hydroxyl group on morphine.[4] Leu-enkephalin has agonistic actions at both the μ- and δ-opioid receptors, with significantly greater preference for the latter. It has little to no effect on the κ-opioid receptor.[5][6]
See also
References
- ↑ "Reduction of spectral interferences using ultraclean gold nanowire arrays in the LDI-MS analysis of a model peptide". Analytical and Bioanalytical Chemistry 406 (19): 4571–83. July 2014. doi:10.1007/s00216-014-7876-7. PMID 24980599.
- ↑ 2.0 2.1 "beta-Lipotropin as a prohormone for the morphinomimetic peptides endorphins and enkephalins". Proceedings of the National Academy of Sciences of the United States of America 73 (6): 2156–9. June 1976. doi:10.1073/pnas.73.6.2156. PMID 1064883. Bibcode: 1976PNAS...73.2156L.
- ↑ "The distribution of methionine-enkephalin and leucine-enkephalin in the brain and peripheral tissues. 1977". British Journal of Pharmacology 120 (4 Suppl): 428–36; discussion 426–7. February 1997. doi:10.1111/j.1476-5381.1997.tb06829.x. PMID 9142421.
- ↑ "Evidence for topographical analogy between methionine-enkephalin and morphine derivatives". Biochemistry 16 (9): 1831–8. May 1977. doi:10.1021/bi00628a011. PMID 870028.
- ↑ "Roles of mu, delta and kappa opioid receptors in spinal and supraspinal mediation of gastrointestinal transit effects and hot-plate analgesia in the mouse". The Journal of Pharmacology and Experimental Therapeutics 230 (2): 341–8. August 1984. PMID 6086883.
- ↑ "Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors". Molecular Pharmacology 45 (2): 330–4. February 1994. PMID 8114680.
 |

