Chemistry:Bexlosteride
From HandWiki
Short description: Chemical compound
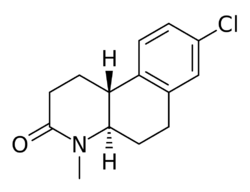 | |
| Clinical data | |
|---|---|
| Other names | LY 300502 |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C14H16ClNO |
| Molar mass | 249.74 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Bexlosteride is a potent and noncompetitive inhibitor of the enzyme 5α-reductase related to finasteride and dutasteride.[1][2] It is selective for the type I isoform of the enzyme.[1] It advanced to Phase III clinical trials, but development was halted at that stage, and it was never marketed.[3][4]
See also
- 5α-Reductase inhibitor
References
- ↑ 1.0 1.1 Androgens and androgen receptor : mechanisms, functions, and clinical application. Boston: Kluwer Academic Publishers. 2002. ISBN 1-4020-7188-4. https://books.google.com/books?id=ODBLQc2BdDIC&q=Bexlosteride&pg=PA167.
- ↑ Strategies for Organic Drug Synthesis and Design. New York: Wiley-Interscience. 2008. ISBN 978-0-470-19039-5. https://books.google.com/books?id=fEwl6Qev-mUC&q=Bexlosteride&pg=PA208.
- ↑ "Drug Profile: Bexlosteride". Adis Insight. http://adisinsight.springer.com/drugs/800009929.
- ↑ Reaxys entry for bexlosteride: Reaxys Registry Number: 6635310
{{Navbox
| name = Drugs used in benign prostatic hypertrophy | title = Drugs used in [[Medicine:Benign prostatic hyperpbenign prostatic hyperplasia (G04C) | state = collapsed | listclass = hlist
| group1 = 5α-Reductase inhibitors | list1 =
| group2 = Alpha-1 blockers | list2 =
| group3 = Steroidal antiandrogens | list3 =
| group4 = Herbal products | list4 =
| group5 = Others | list5 =
}}
 |

