Chemistry:Hexahydrocannabinol
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
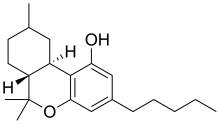
| Formula | C21H32O2 |
| Molar mass | 316.485 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Hexahydrocannabinol (HHC) is a hydrogenated derivative of tetrahydrocannabinol (THC). It is a naturally occurring phytocannabinoid that has rarely been identified as a trace component in Cannabis sativa,[1][2] but can also be produced synthetically by hydrogenation of cannabis extracts.[3] The synthesis and bioactivity of HHC was first reported in 1940 by Roger Adams using tetrahydrocannabinol prepared from cannabidiol.[4]
HHC is a psychoactive substance with effects reportedly similar to that of THC.[5] HHC vaporizers have been openly sold at head shops and convenience stores since at least the early 2020s in North America and Europe. [6][7]
Chemistry
Several research groups have successfully synthesized (+)-HHC and (-)-HHC using citronellal and olivetol,[8] as well as other related compounds.[9] The Elucidation of HHC and related hydrogenated cannabinoid epimers were elucidated using various NMR spectroscopic techniques (ie, NOSY, COSY, 1H) and the diasteromers were isolated using LC-MS and SCFC.[10] While similar compounds have previously been identified in cannabis,[11] hexahydrocannabinol itself has rarely been isolated from the plant. The de Las Heras group in 2020 took lipid extract from Cannabis sativa seeds and discovered 43 cannabinoids in the crude extract; one of them being hexahydrocannabinol. It has two diastereomers at the methyl (9) position. HHC is typically made from CBD. There are no double bonds in the cyclohexyl ring like D8/D9 have—they have been removed from the structure and hydrogens have been added to the compound.[12][13] Similar structural analogs of HHC have been demonstrated to bind to the CB1 receptor and produce cannabinoid effects in animals, with the 9β-HHC enantiomer being much more active than 9α-HHC.[14] While HHC has been shown to bind to the CB1 receptor, it binds with weaker affinity than THC, which has typically been an indication that it is not as intoxicating as typical THC.
Several structurally related HHC analogs have been found to be naturally occurring in Cannabis including cannabiripsol,[15] 9α-hydroxyhexahydrocannabinol, 7-oxo-9α-hydroxyhexa-hydrocannabinol, 10α-hydroxyhexahydrocannabinol, 10aR-hydroxyhexahydrocannabinol and 1′S-hydroxycannabinol,[11] 10α-hydroxy-Δ(9,11)-hexahydrocannabinol and 9β,10β-epoxyhexahydrocannabinol.[16][17]
HHC itself has been found as a degradation byproduct of THC in a similar way that Cannabinol and Delta-8-THC can be formed by the Cannabis plant from Delta-9-THC degradation. The degradation of D9-THC that forms HHC is the reduction of the double carbon bonds that would typically make up the delta isomer position on THCs structure.[18][19]
Delta-9-THC was discovered to partly metabolize into 11-Hydroxy-THC and alpha,10 alpha-epoxy-hexahydrocannabinol along with 1,2-epoxy-hexahydrocannabinol.[20] Cannabidiol was discovered to partly metabolize into 9α-hydroxy-HHC and 8-hydroxy-iso-HHC inside the body. In the presence of alcohol, the methoxy or ethoxy analogs such as 9-methoxy-HHC, 10-methoxy-HHC, 9-ethoxy-HHC and 10-ethoxy-HHC can be formed.[21]
Hexahydrocannabinol should not be confused with the related compounds 9-Nor-9β-hydroxyhexahydrocannabinol (9-Nor-9Beta-HHC) or 9-Hydroxyhexahydrocannabinol (9-OH-HHC) or 11-Hydroxyhexahydrocannabinol (11-OH-HHC and 7-OH-HHC), all of which have also sometimes been referred to as "HHC".[citation needed]
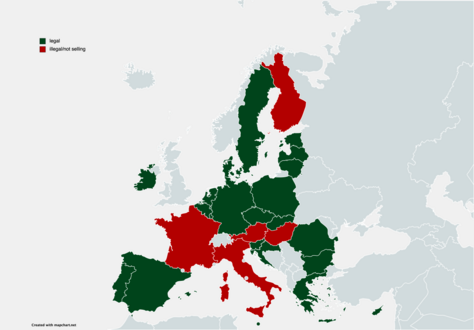
Legality
The ANSM announced the ban (production, sale and use) of HHC and two of its derivatives, HHC-acetate (HHCO) and hexahydrocannabiphorol (HHCP), on France territory from June 13, 2023.[22][23]
In the United Kingdom , HHC would likely be considered illegal under the Psychoactive Substances Act 2016.
Several European countries (Denmark , Belgium, Austria) also recently[when?] banned the sale of HHC.[23]
HHC has been banned in Sweden since July 11, 2023, and in Italy since July 28, 2023.[24] HHC has been banned in Lithuania since November 23, 2022.
HHC has been banned in Slovenia since November 15th, 2023.[25]
The German expert committee for narcotics has, in a meeting on December 4, 2023, suggested to add HHC to the NpSG annex.[26] This recommendation has to date not been enacted by the Federal Government, although this is likely to occur in February of 2024.
See also
- 8-Hydroxyhexahydrocannabinol
- 11-Hydroxyhexahydrocannabinol
- 7,8-Dihydrocannabinol
- Cannabinol
- 9-Nor-9β-hydroxyhexahydrocannabinol
- 9-Hydroxyhexahydrocannabinol
- H4-CBD
- Hexahydrocannabihexol
- HU-243
- Canbisol
- Delta-8-THC
- Delta-10-THC
- Delta-11-THC
- Tetrahydrocannabiphorol
- THC-O-acetate
- Delta-6-Cannabidiol
References
- ↑ "Phytocannabinoids: a unified critical inventory". Natural Product Reports 33 (12): 1357–1392. November 2016. doi:10.1039/c6np00074f. PMID 27722705.
- ↑ "GC-MS Metabolite Profile and Identification of Unusual Homologous Cannabinoids in High Potency Cannabis sativa". Planta Medica 86 (5): 338–347. March 2020. doi:10.1055/a-1110-1045. PMID 32053835.
- ↑ Scialdone MA, "Hydrogenation of cannabis oil", US patent 9694040, issued 10 November 2016, assigned to Research Grow Labs.
- ↑ "Structure of Cannabidiol. VI. Isomerization of Cannabidiol to Tetrahydrocannabinol, a Physiologically Active Product. Conversion of Cannabidiol to Cannabinol". Journal of the American Chemical Society 62 (9): 2402–2405. September 1940. doi:10.1021/ja01866a040.
- ↑ "Saturated Cannabinoids: Update on synthesis strategies and biological studies of these emerging cannabinoid analogs.". ChemRxiv. 3 August 2023. doi:10.26434/chemrxiv-2023-3hk1c.
- ↑ European Monitoring Centre for Drugs and Drug Addiction. (2023). Technical Report: Hexahydrocannabinol (HHC) and related substances. European Monitoring Centre for Drugs and Drug Addiction. doi:10.2810/852912. https://www.emcdda.europa.eu/publications/technical-reports/hhc-and-related-substances_en.
- ↑ "Hexahydrocannabinol and closely related semi-synthetic cannabinoids: A comprehensive review". Drug Testing and Analysis. 2023. doi:10.1002/dta.3519. PMID 37269160.
- ↑ "Efficient one-pot synthetic approaches for cannabinoid analogues and their application to biologically interesting (-)-hexahydrocannabinol and (+)-hexahydrocannabinol". Tetrahedron Letters 49: 3283. 2008. doi:10.1016/j.tetlet.2008.03.075.
- ↑ "Enantioselective Total Synthesis of Potent 9β-11-Hydroxyhexahydrocannabinol". The Journal of Organic Chemistry 85 (2): 1291–1297. January 2020. doi:10.1021/acs.joc.9b02962. PMID 31833372.
- ↑ "Synthesis and Characterization of the Diastereomers of HHC and H4CBD" (in en). Natural Product Communications 18 (3): 1934578X2311589. 2023. doi:10.1177/1934578X231158910. ISSN 1934-578X.
- ↑ 11.0 11.1 "Minor oxygenated cannabinoids from high potency Cannabis sativa L". Phytochemistry 117: 194–199. September 2015. doi:10.1016/j.phytochem.2015.04.007. PMID 26093324. Bibcode: 2015PChem.117..194A.
- ↑ "In vitro metabolism of the equatorial C11-methyl isomer of hexahydrocannabinol in several mammalian species". Drug Metabolism and Disposition 19 (3): 714–716. May 1991. PMID 1680642. http://dmd.aspetjournals.org/cgi/pmidlookup?view=long&pmid=1680642.
- ↑ "Comparative in vitro metabolism of the cannabinoids". Pharmacology, Biochemistry, and Behavior 40 (3): 533–540. November 1991. doi:10.1016/0091-3057(91)90359-a. PMID 1806943.
- ↑ "The importance of the orientation of the C9 substituent to cannabinoid activity". Journal of Medicinal Chemistry 32 (7): 1630–1635. July 1989. doi:10.1021/jm00127a038. PMID 2738895.
- ↑ "Cannabiripsol: a novel Cannabis constituent". Experientia 35 (10): 1278–1279. October 1979. doi:10.1007/BF01963954. PMID 499397.
- ↑ "Isolation and Pharmacological Evaluation of Minor Cannabinoids from High-Potency Cannabis sativa". Journal of Natural Products 78 (6): 1271–1276. June 2015. doi:10.1021/acs.jnatprod.5b00065. PMID 26000707.
- ↑ Peter, Alexendra. "HHC (Hexahydrocannabinol)". Happy420. https://happy-420.de/collections/hhc.
- ↑ "Constituents of Cannabis sativa L. IV. Stability of cannabinoids in stored plant material". Journal of Pharmaceutical Sciences 62 (10): 1601–1605. October 1973. doi:10.1002/jps.2600621005. PMID 4752104.
- ↑ "Stability of tetrahydrocannabinols II". Journal of Pharmaceutical Sciences 67 (1): 27–32. January 1978. doi:10.1002/jps.2600670108. PMID 22740.
- ↑ "Cytochrome P-450 isozymes involved in the oxidative metabolism of delta 9-tetrahydrocannabinol by liver microsomes of adult female rats". Drug Metabolism and Disposition 20 (1): 79–83. January 1992. PMID 1347001. http://dmd.aspetjournals.org/cgi/pmidlookup?view=long&pmid=1347001.
- ↑ "Conversion of Cannabidiol (CBD) into Psychotropic Cannabinoids Including Tetrahydrocannabinol (THC): A Controversy in the Scientific Literature". Toxics 8 (2): 41. June 2020. doi:10.3390/toxics8020041. PMID 32503116.
- ↑ "Cannabis : le HHC sera interdit en France à partir de mardi" (in fr). Le Monde.fr. 2023-06-12. https://www.lemonde.fr/sante/article/2023/06/12/cannabis-le-hhc-sera-interdit-en-france-a-partir-de-mardi_6177293_1651302.html.
- ↑ 23.0 23.1 "Actualité - L'ANSM classe l'hexahydrocannabinol (HHC) et deux de ses dérivés sur la liste des stupéfiants" (in fr). https://ansm.sante.fr/actualites/lansm-classe-lhexahydrocannabinol-hhc-et-deux-de-ses-derives-sur-la-liste-des-stupefiants.
- ↑ "Gazzetta Ufficiale". https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2023-07-25&atto.codiceRedazionale=23A04164&elenco30giorni=true&fbclid=IwAR0d8pv68emAlSwx5PPesHph7DtpXGIfcfaIaSbHuNDnEcBWRZf4ws750Ag.
- ↑ "eUprava - Predlog predpisa". https://e-uprava.gov.si/si/drzava-in-druzba/e-demokracija/predlogi-predpisov/predlog-predpisa.html?id=15805.
- ↑ "Sachverständigenausschuss für Betäubungsmittel nach § 1 Abs. 2 BtMG und Neue-psychoaktive-Stoffe nach § 7 NpSG" (in de). https://www.bfarm.de/DE/Bundesopiumstelle/Betaeubungsmittel/Sachverstaendigenausschuss/Sitzungen/Ergebnisse_59.html?nn=595366.
 |