Chemistry:Ifenprodil
| |
| Names | |
|---|---|
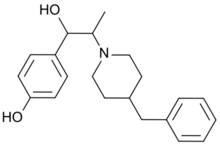
| IUPAC name
4-[2-(4-Benzylpiperidin-1-yl)-1-hydroxypropyl]phenol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H27NO2 | |
| Molar mass | 325.445 |
| Pharmacology | |
| 1=ATC code }} | C04AX28 (WHO) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ifenprodil is an inhibitor of the NMDA receptor,[1] specifically of GluN1 (glycine-binding NMDA receptor subunit 1) and GluN2B (glutamate-binding NMDA receptor subunit 2) subunits.[2] Additionally, ifenprodil inhibits GIRK channels, and interacts with alpha1 adrenergic, serotonin, and sigma receptors.[3]
NMDA receptors are multimeric ionotropic glutamate receptors composed of four subunits. GluN1 is obligate for functional expression. Other subunits include GluN2A, GluN2B, and the more recently discovered GluN3 subunits. Ifenprodil selectively inhibits NMDA receptors containing the GluN2B subunit.
As ifenprodil tartrate, it has been marketed in some countries, including Japan and France, as a cerebral vasodilator, under the trade names Cerocral, Dilvax, and Vadilex.[4]
Ifenprodil has been studied as a possible medication to prevent tinnitus after acoustic trauma.[5]
It is currently in phase III clinical trials to treat SARS-CoV2 infection and phase II trials for idiopathic pulmonary fibrosis.[6]
References
- ↑ "Ifenprodil is a novel type of N-methyl-D-aspartate receptor antagonist: interaction with polyamines". Mol. Pharmacol. 36 (5): 758–65. 1989. PMID 2555674.
- ↑ "Neurosteroid modulation of N-methyl-d-aspartate receptors: Molecular mechanism and behavioral effects". Steroids 76 (13): 1409–18. Dec 2011. doi:10.1016/j.steroids.2011.09.002. PMID 21925193.
- ↑ Kobayashi, Toru; Washiyama, Kazuo; Ikeda, Kazutaka (3 August 2005). "Inhibition of G Protein-Activated Inwardly Rectifying K+ Channels by Ifenprodil". Neuropsychopharmacology 31 (3): 516–524. doi:10.1038/sj.npp.1300844. PMID 16123769.
- ↑ The Merck Index (Thirteenth ed.). Whitehouse Station, NJ: Merck Research Laboratories Division of Merck & Co., Inc.. 2001. p. 878.
- ↑ Guitton, Matthieu J.; Dudai, Yadin (2007). "Blockade of Cochlear NMDA Receptors Prevents Long-Term Tinnitus during a Brief Consolidation Window after Acoustic Trauma". Neural Plasticity (Hindawi Limited) 2007: 1–11. doi:10.1155/2007/80904. ISSN 2090-5904. PMID 18301716.
- ↑ "Ifenprodil - Algernon Pharmaceuticals - AdisInsight". https://adisinsight.springer.com/drugs/800055643.
 |

