Chemistry:Δ-8-Tetrahydrocannabinol
| |
| |
| Names | |
|---|---|
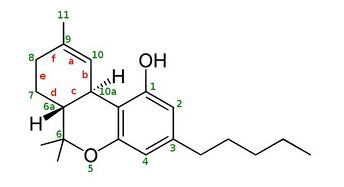
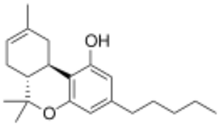
| IUPAC name
6,6,9-trimethyl-3-pentyl-6a,7,10,10a-tetrahydrobenzo[c]chromen-1-ol
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H30O2 | |
| Molar mass | 314.5 g/mol |
| Density | 1.0±0.1 g/cm3 |
| Boiling point | 383.5±42.0 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Δ-8-tetrahydrocannabinol (delta-8-THC, Δ8-THC)[1] is a psychoactive cannabinoid found in the cannabis plant.[2][3][4] It is an isomer of delta-9-tetrahydrocannabinol (delta-9-THC, Δ9-THC), the compound commonly known as THC.
Effects
∆8-THC is moderately less potent than Δ9-THC.[5][6] This means that while its effects are similar to those of Δ9-THC, it would take more ∆8-THC to achieve a comparable level of effect.[7] ∆8-THC and Δ9-THC both contain a double bond in their molecular chain, but the location is different. In ∆8-THC, the double bond is in the eighth carbon chain, while in Δ9-THC, the double bond is in the ninth.
A 1973 study testing the effects of ∆8-THC in dogs and monkeys reported that a single oral dose of 9,000 milligrams per kilogram of body mass (mg/kg) was nonlethal in all dogs and monkeys studied.[8][9] The same study reported that the median lethal dose of ∆8-THC in rats was comparable to that of ∆9-THC.[8] Both isomers of THC have been found to cause a transient increase in blood pressure in rats,[10] although the effects of cannabinoids on the cardiovascular system are complex.[11] Animal studies indicate that ∆8-THC exerts many of its central effects by binding to cannabinoid receptors found in various regions of the brain, including the cerebral cortex, thalamus, basal ganglia, hippocampus, and cerebellum.[12][13]
Pharmacology
Pharmacodynamics
The pharmacodynamic profile of ∆8-THC is similar to that of ∆9-THC.[5][6] It is a partial agonist of CB1 and CB2 cannabinoid receptors with about half the potency of ∆9-THC in most but not all measures of biological activity.[14][15][16] ∆8-THC has been reported to have a Ki value of 44 ± 12 nM at the CB1 receptor and 44 ± 17 nM at the CB2 receptor.[17] These values are higher than those typically reported for ∆9-THC (CB1 Ki = 40.7 nM) at the same receptors, indicating that ∆8-THC binds to cannabinoid receptors less efficiently than ∆9-THC.[18]
Pharmacokinetics
The pharmacokinetic profile of ∆8-THC is also similar to that of ∆9-THC.[5][6] Following ingestion in humans, hepatic cytochrome P450 enzymes including CYP2C9 and CYP3A4 first convert ∆8-THC into 11-hydroxy-Δ8-tetrahydrocannabinol (11-OH-Δ8-THC).[19][20] Next, dehydrogenase enzymes convert 11-OH-Δ8-THC into 11-nor-Δ8-tetrahydrocannabinol-9-carboxylic acid (11-nor-Δ8-THC-9-COOH, also known as Δ8-THC-11-oic acid).[20][21] Finally, Δ8-THC-11-oic acid undergoes glucuronidation by glucuronidase enzymes to form 11-nor-Δ8-tetrahydrocannabinol-9-carboxylic acid glucuronide (Δ8-THC-COOH-glu),[20][21] which is then excreted in the urine.[22][23]
Physical and chemical properties
∆8-THC is a tricyclic terpenoid. Although it has the same chemical formula as ∆9-THC, one of its carbon-carbon double bonds is located in a different position.[5] This difference in structure increases the chemical stability of ∆8-THC relative to ∆9-THC, lengthening shelf life and allowing the compound to resist undergoing oxidation to cannabinol over time.[14] Like other cannabinoids, ∆8-THC is very lipophilic (log P = 7.4[24]). It is an extremely viscous, colorless oil at room temperature.[25]
While ∆8-THC is naturally found in plants of the Cannabis genus,[4] this compound can also be produced in an industrial or laboratory setting by exposing CBD to acids and heat.[26][27][28] Solvents that may be used during this process include methylene chloride, toluene, and hexane.[28] Various Brønsted or Lewis acids that may be used to facilitate this isomerization include tosylic acid, indium(III) triflate, trimethylsilyl trifluoromethanesulfonate, hydrochloric acid, and sulfuric acid.[28][29] Because it is possible for chemical contaminants to be generated during the process of converting CBD to ∆8-THC, such as Δ10-THC, 9-OH-HHC and other side products, as well as the potentially toxic chemical reagents used during manufacture, concern has been raised about the safety of untested or impure ∆8-THC products.[29][30]
The ongoing controversy regarding the legal status of ∆8-THC in the U.S. is complicated by chemical nomenclature. According to a 2019 literature review published in Clinical Toxicology, the term synthetic cannabinoid typically refers to a full agonist of CB1 and CB2 cannabinoid receptors.[31] According to the review, the following is stated:
"The psychoactive (and probably the toxic) effects of synthetic cannabinoid receptor agonists are likely due to their action as full receptor agonists and their greater potency at CB1 receptors."
However, ∆8-THC and ∆9-THC are partial agonists of cannabinoid receptors.[15] They are less potent and less toxic than many synthetic cannabinoids.[32] It has not been definitively proven if full agonism is the reason for toxicity since ∆9-THC has been shown to act as a full CB1 agonist on specific CB1 receptors located in the hippocampus section of the brain.[33] Furthermore, the synthetic cannabinoid EG-018 acts as a partial agonist.[34] The classical cannabinoid structure is that of a dibenzopyran structure. This group includes THC. THC interacts with a different spot inside of the CB1 receptor than synthetic cannabinoid such JWH-018. This may explain the latter's toxicity.[35]
History
The partial synthesis of ∆8-THC was published in 1941 by Roger Adams and colleagues at the University of Illinois.[36] In 1942, the same research group studied its physiological and psychoactive effects after oral dosing in human volunteers.[37] Total syntheses of ∆8-THC were achieved by 1965.[38] In 1966, the chemical structure of ∆8-THC isolated from cannabis was characterized using modern methods by Richard L. Hively, William A. Mosher, and Friedrich W. Hoffmann at the University of Delaware.[39] A stereospecific synthesis of ∆8-THC from olivetol and verbenol was reported by Raphael Mechoulam and colleagues at the Weizmann Institute of Science in 1967.[40] ∆8-THC was often referred to as "Delta-6-THC" (Δ6-THC) in early scientific literature, but this name is no longer conventional among most authors.[41]
Legality in the United States
In 1937, ∆9-THC was effectively made illegal with the passage of the (since-repealed) Marihuana Tax Act, which made growing cannabis require a tax stamp. The Anti-Drug Abuse Act of 1986 re-enacted mandatory minimum sentences for cannabis-related offenses.[42] As of 1 September 2023, 23 states have decriminalized cannabis, with others having reduced penalties.
The 2018 United States farm bill, signed into law in December 2018, states the following:
"The term hemp means the plant Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis."
∆8-THC products partially synthesized from compliant sources (including industrial hemp and derivative cannabidiol extracts) experienced a rise in use following the passage of the bill.[43] This led to it being sold by retailers, including head shops, smoke shops, vape shops, dispensaries, gas stations, and convenience stores.[44]
Common products range from bulk quantities of unrefined distillate to prepared edibles and atomizer cartridges.[45][46] They are usually marketed as federally legal alternatives to their ∆9-THC counterparts.[47] However, the legal status of ∆8-THC at the federal level is in question with some believing that the October 2020 DEA Interim Final Rule addressing "synthetics" applied to ∆8-THC products and other hemp derivatives allowed by the Farm Bill.[48][49] While most states have not arrested significant numbers of people for ∆8-THC, some have been arrested and charged, leading to confusion as to its legal status in those states.[50][51][52][53]
Despite claims of legality by manufacturers, independent testing of products from retail has revealed significant levels of ∆9-THC, many of which are above the legal threshold,[54] although synthetic ∆9-THC (dronabinol), when in FDA-approved drug products with a currently accepted medical use in the United States, such as Syndros or Marinol, is under Schedule II or III of the Controlled Substances Act, respectively.[55][56]
∆8-THC products have been sold in licensed, regulated recreational cannabis and medical cannabis industries within the United States including California[57] and Pennsylvania's licensed, regulated medical cannabis system since 2020.[58] The states of Michigan and Oregon have regulated delta-8-THC products sold under state cannabis rulings.[59][60]
∆8-THC has not been evaluated or approved by the FDA. Consequently, Δ-8-tetrahydrocannabinol is not recognized under the Federal Food, Drug, and Cosmetic Act as safe and effective for any use.[61] The FDA has taken action against businesses that have illegally marketed ∆8-THC for therapeutic use.[62] The FDA has also taken action against businesses that sold ∆8-THC in forms that closely resemble (typically non-psychoactive) food products such as chips or cookies.[63]
Safety
∆8-THC is typically synthesized from cannabidiol extracted from hemp,[64] as the natural quantities of ∆8-THC found in hemp are low. The reaction often yields a mixture that contains other cannabinoids and unknown reaction by-products. As a result, most products sold as ∆8-THC are not actually pure ∆8-THC.[64] Little is known about the identity and the health effects of the impurities.[64] Some manufacturers of ∆8-THC may use household chemicals in the synthesis process, potentially introducing harmful contaminants.[65] The safety profile of regular, long-term delta-8-THC use is unknown.[66]
There have been at least 104 adverse event reports made regarding ∆8-THC,[65] and at least two deaths associated with ∆8-THC products.[67][68] US national poison control centers received 2,362 exposure cases of delta-8 THC products between January 1, 2021, and February 28, 2022; 58% of these exposures involved adults, and 70% required medical care.[65]
Research
Although it is a minor constituent of medical cannabis, no large clinical studies have been conducted on delta-8-THC alone as of 2022.[69] There is one study (estimated to be complete by June 2025), that is focused on determining the degree of pharmacologic and pharmacokinetic similarity between ∆8-THC and ∆9-THC.[70]
See also
- Ajulemic acid
- Cannabinoid
- Cannabis (drug)
- delta-3-Tetrahydrocannabinol
- delta-4-Tetrahydrocannabinol
- delta-7-Tetrahydrocannabinol (delta-5-tetrahydrocannabinol)
- delta-10-Tetrahydrocannabinol (delta-2-tetrahydrocannabinol)
- delta-6-Cannabidiol
- 11-Hydroxy-Delta-8-THC
- Endocannabinoid system
- Hexahydrocannabinol
- 7,8-Dihydrocannabinol
- Tetrahydrocannabinol
- Tetrahydrocannabutol
- Tetrahydrocannabiphorol
- THC-O-acetate
- THC-O-phosphate
References
- ↑ Smith, Dana (25 July 2022). "How Delta-8 THC Works, and Why Experts Are Worried About It - This popular cannabis product claims to be milder than regular marijuana. But is it legal? And is it safe? + Comment". The New York Times. https://www.nytimes.com/2022/07/01/well/mind/delta-8-thc-marijuana.html#permid=122220022.
- ↑ "NCI Drug Dictionary" (in en). 2 February 2011. https://www.cancer.gov/publications/dictionaries/cancer-drug.
- ↑ "Δ8-Tetrahydrocannabinol". U.S. Secretary of Commerce. https://webbook.nist.gov/cgi/inchi?ID=C5957755&Mask=200.
- ↑ 4.0 4.1 "Development and Optimization of Supercritical Fluid Extraction Setup Leading to Quantification of 11 Cannabinoids Derived from Medicinal Cannabis". Biology 10 (6): 481. May 2021. doi:10.3390/biology10060481. PMID 34071473.
- ↑ 5.0 5.1 5.2 5.3 "Chemistry and Structure-Activity Relationships of Cannabinoids: An Overview". The Cannabinoids: Chemical, Pharmacologic, and Therapeutic Aspects. 1984. pp. 63–78. doi:10.1016/b978-0-12-044620-9.50009-9. ISBN 978-0-12-044620-9. https://books.google.com/books?id=x7y7ruiagigC&pg=PA63.
- ↑ 6.0 6.1 6.2 "Delta-8- and delta-9-tetrahydrocannabinol comparison in man by oral and intravenous administration". Clinical Pharmacology and Therapeutics 14 (3): 353–7. May 1973. doi:10.1002/cpt1973143353. PMID 4698563.
- ↑ "delta-8-tetrahydrocannabinol" (in en). 2011-02-02. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/delta-8-tetrahydrocannabinol.
- ↑ 8.0 8.1 "Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys". Toxicology and Applied Pharmacology 25 (3): 363–72. July 1973. doi:10.1016/0041-008x(73)90310-4. PMID 4199474.
- ↑ "ChemIDplus - 0005957755 - HCAWPGARWVBULJ-IAGOWNOFSA-N - delta-8-Tetrahydrocannabinol - Similar structures search, synonyms, formulas, resource links, and other chemical information." (in en). https://chem.nlm.nih.gov/chemidplus/rn/5957-75-5.
- ↑ "Vasoconstrictor actions of delta8- and delta9-tetrahydrocannabinol in the rat". The Journal of Pharmacology and Experimental Therapeutics 196 (3): 649–56. March 1976. PMID 4606. https://jpet.aspetjournals.org/content/196/3/649.short.
- ↑ "A Systematic Review of the Complex Effects of Cannabinoids on Cerebral and Peripheral Circulation in Animal Models". Frontiers in Physiology 9: 622. 29 May 2018. doi:10.3389/fphys.2018.00622. PMID 29896112.
- ↑ "PET studies in the primate brain and biodistribution in mice using (-)-5'-18F-delta 8-THC". Pharmacology, Biochemistry, and Behavior 40 (3): 503–7. November 1991. doi:10.1016/0091-3057(91)90354-5. PMID 1666914.
- ↑ "Effects of cannabinoids on levels of acetylcholine and choline and on turnover rate of acetylcholine in various regions of the mouse brain". Alcohol and Drug Research 7 (5–6): 525–32. 1987. INIST:7401152. PMID 3620017.
- ↑ 14.0 14.1 "An efficient new cannabinoid antiemetic in pediatric oncology". Life Sciences 56 (23–24): 2097–102. May 1995. doi:10.1016/0024-3205(95)00194-b. PMID 7776837.
- ↑ 15.0 15.1 "Cannabinoids and neuroinflammation". British Journal of Pharmacology 141 (5): 775–85. March 2004. doi:10.1038/sj.bjp.0705667. PMID 14757702.
- ↑ "Molecular Targets of the Phytocannabinoids: A Complex Picture". Phytocannabinoids. Progress in the Chemistry of Organic Natural Products. 103. Springer. 2017. pp. 103–131. doi:10.1007/978-3-319-45541-9_4. ISBN 978-3-319-45539-6.
- ↑ "The Structure-Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation". Perspectives in Medicinal Chemistry 8: 17–39. January 2016. doi:10.4137/PMC.S32171. PMID 27398024.
- ↑ "The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin". British Journal of Pharmacology 153 (2): 199–215. January 2008. doi:10.1038/sj.bjp.0707442. PMID 17828291.
- ↑ "Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review". Drug Metabolism Reviews 46 (1): 86–95. February 2014. doi:10.3109/03602532.2013.849268. PMID 24160757. https://zenodo.org/record/1093138.
- ↑ 20.0 20.1 20.2 "GC/MS Determination of 11-Nor-9-Carboxy-Δ 8 -tetrahydrocannabinol in Urine from Cannabis Users". Analytical Letters 31 (15): 2635–2643. December 1998. doi:10.1080/00032719808005332.
- ↑ 21.0 21.1 "LC-MS method for the estimation of delta8-THC and 11-nor-delta8-THC-9-COOH in plasma". Journal of Pharmaceutical and Biomedical Analysis 38 (1): 112–8. June 2005. doi:10.1016/j.jpba.2004.11.055. PMID 15907628.
- ↑ "Comparative in vitro metabolism of the cannabinoids". Pharmacology, Biochemistry, and Behavior 40 (3): 533–40. November 1991. doi:10.1016/0091-3057(91)90359-A. PMID 1806943.
- ↑ "Delta 6-tetrahydrocannabinol-7-oic acid, a urinary delta 6-THC metabolite: isolation and synthesis". Experientia 29 (10): 1193–5. October 1973. doi:10.1007/BF01935065. PMID 4758913.
- ↑ "Characterization of the lipophilicity of natural and synthetic analogs of ∆9-THC and its relationship to pharmacological potency". The Journal of Pharmacology and Experimental Therapeutics 255 (2): 624–30. November 1990. PMID 2173751. https://jpet.aspetjournals.org/content/255/2/624.short.
- ↑ "Oral and parenteral formulations of marijuana constituents". Journal of Pharmaceutical Sciences 61 (7): 1106–12. July 1972. doi:10.1002/jps.2600610715. PMID 4625586.
- ↑ "Conversion of Cannabidiol (CBD) into Psychotropic Cannabinoids Including Tetrahydrocannabinol (THC): A Controversy in the Scientific Literature". Toxics 8 (2): 41. June 2020. doi:10.3390/toxics8020041. PMID 32503116.
- ↑ "Hashish—VII". Tetrahedron 22 (4): 1481–1488. January 1966. doi:10.1016/S0040-4020(01)99446-3.
- ↑ 28.0 28.1 28.2 "Cannabidiol as the Substrate in Acid-Catalyzed Intramolecular Cyclization". Journal of Natural Products 83 (10): 2894–2901. October 2020. doi:10.1021/acs.jnatprod.0c00436. PMID 32991167.
- ↑ 29.0 29.1 "Is delta-8 THC safe? Here's what the experts say" (in en-US). 2021-06-09. https://www.leafly.com/news/health/is-delta-8-thc-safe-heres-what-the-experts-say.
- ↑ "Delta-8-THC craze concerns chemists". Chemical & Engineering News 99 (31). 30 August 2021. https://cen.acs.org/biological-chemistry/natural-products/Delta-8-THC-craze-concerns/99/i31.
- ↑ "Synthetic cannabinoid receptor agonists: classification and nomenclature". Clinical Toxicology 58 (2): 82–98. February 2020. doi:10.1080/15563650.2019.1661425. PMID 31524007.
- ↑ "Cannabinoid Toxicity". StatPearls. StatPearls Publishing. 2021. http://www.ncbi.nlm.nih.gov/books/NBK482175/.
- ↑ "Delta9-tetrahydrocannabinol is a full agonist at CB1 receptors on GABA neuron axon terminals in the hippocampus". Neuropharmacology 59 (1–2): 121–7. 2010. doi:10.1016/j.neuropharm.2010.04.013. PMID 20417220.
- ↑ "In vitro and in vivo pharmacological evaluation of the synthetic cannabinoid receptor agonist EG-018". Pharmacology, Biochemistry, and Behavior 193: 172918. June 2020. doi:10.1016/j.pbb.2020.172918. PMID 32247816.
- ↑ "Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indenes". Current Medicinal Chemistry 12 (12): 1395–411. 31 May 2005. doi:10.2174/0929867054020864. PMID 15974991.
- ↑ "Structure of Cannabidiol. XII. Isomerization to Tetrahydrocannabinols 1". Journal of the American Chemical Society 63 (8): 2209–2213. August 1941. doi:10.1021/ja01853a052.
- ↑ "Marihuana: Harvey Lecture, February 19, 1942". Bulletin of the New York Academy of Medicine 18 (11): 705–30. November 1942. PMID 19312292.
- ↑ "Marihuana chemistry". Science 168 (3936): 1159–66. June 1970. doi:10.1126/science.168.3936.1159. PMID 4910003. Bibcode: 1970Sci...168.1159M.
- ↑ "Isolation of trans-delta-tetrahydrocannabinol from marijuana". Journal of the American Chemical Society 88 (8): 1832–3. April 1966. doi:10.1021/ja00960a056. PMID 5942992.
- ↑ "A stereospecific synthesis of (-)-delta 1- and (-)-delta 1(6)-tetrahydrocannabinols". Journal of the American Chemical Society 89 (17): 4552–4. August 1967. doi:10.1021/ja00993a072. PMID 6046550.
- ↑ "Cannabinoid pharmacology: the first 66 years". British Journal of Pharmacology 147 (Suppl 1): S163-71. January 2006. doi:10.1038/sj.bjp.0706406. PMID 16402100.
- ↑ Amanda Reed (2021-12-13). "What the Hell Is Delta-8 THC?" (in en-US). https://www.gearpatrol.com/home/a38426081/what-is-delta-8/.
- ↑ LoParco, Cassidy; Rossheim, Matthew; Walters, Scott; Zhou, Zhengyang; Olsson, Sofia; Sussman, Steve (29 Jan 2023). "Delta-8 tetrahydrocannabinol: a scoping review and commentary". Addiction (Society for the Study of Addiction) 118 (6): 1011–1028. doi:10.1111/add.16142. PMID 36710464.
- ↑ Schaefer, Brittany (10 February 2023). "Attorney General William Tong warns vape shops about delta-8 THC products". Nexstar Media. https://www.wtnh.com/news/attorney-general-william-tong-warns-vape-shops-about-delta-8-thc-products/. "We're seeing delta 8 products being sold across the state, everywhere we went to every vape shop we visited and gas stations as well,"
- ↑ Guo, Weihong; Vrdoljak, Gordon; Liao, Ven-Chi; Moezzi, Bahman (21 June 2021). "Major Constituents of Cannabis Vape Oil Liquid, Vapor and Aerosol in California Vape Oil Cartridge Samples". Frontiers in Chemistry (Front Chem.) 9. doi:10.3389/fchem.2021.694905. PMID 34368078.
- ↑ Tadlock, Collin (7 April 2023). "Cannabis sales have buyers, sellers on a different high". The Charlotte Post. https://www.thecharlottepost.com/news/2023/04/07/local-state/cannabis-sales-have-buyers-sellers-on-a-high/. "Located at vape shops, convenience stores and even gas stations, Delta-8 is well-accessible to consumers. Products are available in different forms, including gummies, chocolate, vaping cartridges, infused drinks and even breakfast cereal."
- ↑ "Delta-8-THC Promises to Get You High Without the Paranoia or Anxiety". Discover Magazine. 23 September 2020. https://www.discovermagazine.com/health/delta-8-thc-promises-to-get-you-high-without-the-paranoia-or-anxiety.
- ↑ "DEA Tried Banning New Cannabis Product, Sellers Still Selling". Dope Magazine. 23 October 2020. https://dopemagazine.com/dea-tried-banning-new-cannabis-product-sellers-still-selling/.
- ↑ "How Some THC Is Legal — For Now". Rolling Stone. 18 January 2021. https://www.rollingstone.com/culture/culture-features/delta-8-thc-legal-weed-explained-1113859/.
- ↑ Stewart, Jeremy (2021-10-13). "Investigation into sale of THC products leads to arrests" (in en). https://www.northwestgeorgianews.com/polk_standard_journal/news/cedartown/investigation-into-sale-of-thc-products-leads-to-arrests/article_8775c126-2c38-11ec-99fd-6f43dc8501cd.html.
- ↑ Rogers, Chase (2021-02-11). "Port Lavaca smoke shop owner turns self in, retains Austin cannabis law attorney" (in en). https://www.victoriaadvocate.com/premium/port-lavaca-smoke-shop-owner-turns-self-in-retains-austin-cannabis-law-attorney/article_48a421b0-6cbf-11eb-9099-c3b02e465657.html.
- ↑ Jones, Kylie (2021-03-23). "Upstate police seize Delta-8 THC products from vape shop, owner argues they're legal" (in en). https://www.wyff4.com/article/clinton-laurens-county-police-seize-delta-8-thc-from-vape-shop-owner-argues-its-legal/35917902.
- ↑ Matsuoka, Sayaka (2021-10-27). "GSO hemp shop owners say police wrongly searched, seized legal THC products" (in en). https://triad-city-beat.com/gso-hemp-raid/.
- ↑ "The Unregulated Distribution And Sale Of Consumer Products Marketed As Delta-8 THC". https://irp.cdn-website.com/6531d7ca/files/uploaded/USCC%20Delta-8%20Kit.pdf.
- ↑ "Schedules of Controlled Substances: Placement of FDA-Approved Products of Oral Solutions Containing Dronabinol [(-)-delta-9-trans-tetrahydrocannabinol (delta-9-THC) in Schedule II"]. Drug Enforcement Administration, Department of Justice. 23 March 2017. https://www.federalregister.gov/documents/2017/03/23/2017-05809/schedules-of-controlled-substances-placement-of-fda-approved-products-of-oral-solutions-containing. Retrieved 18 December 2023.
- ↑ "Schedules of Controlled Substances: Rescheduling of the Food and Drug Administration Approved Product Containing Synthetic Dronabinol [(-)-D9-(trans)-Tetrahydrocannabinol in Sesame Oil and Encapsulated in Soft Gelatin Capsules From Schedule II to Schedule III"]. Drug Enforcement Administration, Department of Justice. 2 July 1999. https://web.archive.org/web/20221104154012/https://www.deadiversion.usdoj.gov/fed_regs/rules/1999/fr0702.htm. Retrieved 18 December 2023.
- ↑ "Korova Korova - Biggie Cookie, 1,000mg". https://weedmaps.com/brands/korova-edibles/products/korova-edibles-biggie-cookie-1-000-mg/reviews.
- ↑ "Delta-8 THC: The lesser known cannabinoid that FarmaceuticalRX is taking on". 14 January 2020. https://www.maitrimedicinals.com/blog/2020/1/14/delta-8-thc-the-lesser-known-cannabinoid-that-farmaceuticalrx-is-taking-on.
- ↑ http://www.legislature.mi.gov/documents/2021-2022/publicact/htm/2021-PA-0056.htm
- ↑ https://olis.oregonlegislature.gov/liz/2021R1/Downloads/MeasureDocument/HB3000/Enrolled
- ↑ Commissioner, Office of the (2022-05-04). "5 Things to Know about Delta-8 Tetrahydrocannabinol – Delta-8 THC" (in en). FDA. https://www.fda.gov/consumers/consumer-updates/5-things-know-about-delta-8-tetrahydrocannabinol-delta-8-thc.
- ↑ Commissioner, Office of the (2022-05-05). "FDA Issues Warning Letters to Companies Illegally Selling CBD and Delta-8 THC Products" (in en). https://www.fda.gov/news-events/press-announcements/fda-issues-warning-letters-companies-illegally-selling-cbd-and-delta-8-thc-products.
- ↑ Commissioner, Office of the (2023-07-06). "FDA, FTC Warn Six Companies for Illegally Selling Copycat Food Products Containing Delta-8 THC" (in en). https://www.fda.gov/news-events/press-announcements/fda-ftc-warn-six-companies-illegally-selling-copycat-food-products-containing-delta-8-thc.
- ↑ 64.0 64.1 64.2 Britt E. Erickson (August 29, 2021). "Delta-8-THC craze concerns chemists: Unidentified by-products and lack of regulatory oversight spell trouble for cannabis products synthesized from CBD". Chemical & Engineering News. https://cendigitalmagazine.acs.org/2021/08/30/delta-8-thc-craze-concerns-chemists-3/content.html.
- ↑ 65.0 65.1 65.2 "5 Things to Know about Delta-8 Tetrahydrocannabinol – Delta-8 THC" (in en). US Food and Drug Administration. 2022-03-25. https://www.fda.gov/consumers/consumer-updates/5-things-know-about-delta-8-tetrahydrocannabinol-delta-8-thc.
- ↑ Dotson, Samuel; Johnson‐Arbor, Kelly; Schuster, Randi M.; Tervo‐Clemmens, Brenden; Evins, A. Eden (2022). "Unknown risks of psychosis and addiction with delta‐8‐THC: A call for research, regulation, and clinical caution" (in en). Addiction 117 (9): 2371–2373. doi:10.1111/add.15873. ISSN 0965-2140. PMID 35322899.
- ↑ "Mother charged with murder after child dies from ingesting delta-8 THC gummies, officials say" (in en). 2022-10-21. https://www.kiro7.com/news/trending/mother-charged-with-murder-after-child-dies-ingesting-delta-8-thc-gummies-officials-say/65QRUSPTUVELHILSVQLSKDKXSM/.
- ↑ Cheng, Brad (2022-12-06). "Minnesota Sues to Halt Sales of FDA-Targeted "Death by Gummy Bears" Edibles" (in en-US). Global Cannabis Times. https://globalcannabistimes.com/minnesota-sues-to-halt-sales-of-fda-investigated-death-by-gummy-bears-edibles/.
- ↑ Tagen, Michael; Klumpers, Linda E. (2022). "Review of delta‐8‐tetrahydrocannabinol (Δ 8 ‐THC): Comparative pharmacology with Δ 9 ‐THC" (in en). British Journal of Pharmacology 179 (15): 3915–3933. doi:10.1111/bph.15865. ISSN 0007-1188. PMID 35523678.
- ↑ "CTG Labs - NCBI". https://clinicaltrials.gov/study/NCT05287256.
External links
 |