Chemistry:2C-T-2
| |
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
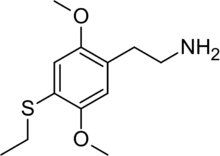
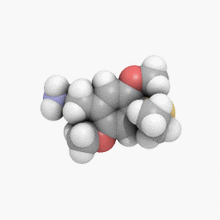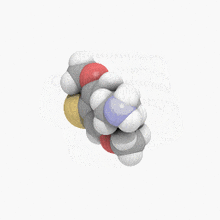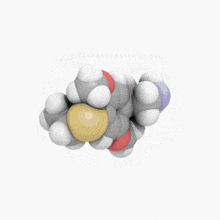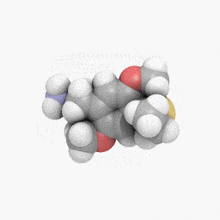
2-[4-(Ethylsulfanyl)-2,5-dimethoxyphenyl]ethan-1-amine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C12H19NO2S | |
| Molar mass | 241.35 g/mol |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302 | |
| P264, P270, P301+312, P330, P501 | |
| Pharmacology | |
| Legal status |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2C-T-2 is a psychedelic and entactogenic phenethylamine of the 2C family.[1] It was first synthesized in 1981 by Alexander Shulgin, and rated by him as one of the "magical half-dozen" most important psychedelic phenethylamine compounds.[2][3] The drug has structural and pharmacodynamic properties similar to those of 2C-T-7 ("Blue Mystic").
Dosage
In Alexander Shulgin's book PiHKAL, the dosage range is listed as 12 to 25 mg.[3]
Pharmacology
The mechanism of action that produces 2C-T-2’s hallucinogenic and entheogenic effects is shown to be most likely a result from action as a 5-HT2A, 5-HT2B, and 5-HT2C serotonin receptor agonist,[4] a mechanism of action shared by the hallucinogenic tryptamines and phenethylamines to varying degrees.[5][6] 2C-T-2 has also shown to be a partial agonist of adrenergic receptors.[7]
Dangers
A potential risk of neurotoxicity from 2C-T-2 use (and 2C chemical series in general) has been shown in serotonergic and dopaminergic containing neurons.[8] This has also been shown to be magnified in serotonergic-containing cells with combined use of 2C series drugs with alcohol, MDMA, and methamphetamine.[9]
Severe 'intoxication' on 2C series drugs has been observed as behavior that includes: intense hallucinations, agitation, aggression, violence, dysphoria, hypertension, tachycardia, seizures, and hyperthermia.[10]
Drug prohibition laws
Argentina
2C-T-2 is also a controlled substance in Argentina as well as 2C-B and 2C-I.[11]
Canada
As of October 31, 2016, 2C-T-2 is a controlled substance (Schedule III) in Canada.[12]
China
As of October 2015 2C-T-2 is a controlled substance in China.[13]
Netherlands
The Netherlands became the first country in the world to ban 2C-T-2, and classify it as a hard drug, by law. In April, 1999, 2C-T-2 became a list I drug of the Opium Law.
Sweden
Schedule I in Sweden.
2C-T-2 was first classified as "health hazard" under the act Lagen om förbud mot vissa hälsofarliga varor (translated Act on the Prohibition of Certain Goods Dangerous to Health) as of April 1, 1999, under SFS 1999:58[14] that made it illegal to sell or possess.
The Riksdag added 2C-T-2 to Narcotic Drugs Punishments Act under Swedish schedule I ("substances, plant materials and fungi which normally do not have medical use") as of March 16, 2004, published by Medical Products Agency (MPA) in regulation LVFS 2004:3 listed as 2C-T-2, 2,5-dimetoxi-4-etyltiofenetylamin.[15]
United Kingdom
2C-T-2 and all other compounds featured in PiHKAL are illegal drugs in the United Kingdom .
United States
2C-T-2 is specifically listed as a schedule I substance under SEC. 1152 of S.3187: Food and Drug Administration Safety and Innovation Act of 2012.[16]
Australia
2C-T-2 is considered a Schedule 9 prohibited substance in Australia under the Poisons Standard (October 2015).[17] A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities.[17]
References
- ↑ "Stolaroff's & Well's Study". 2001-02-06. https://erowid.org/chemicals/2ct7/article1/stolaroff.shtml.
- ↑ "New designer drug 2,5-dimethoxy-4-ethylthio-beta-phenethylamine (2C-T-2): studies on its metabolism and toxicological detection in rat urine using gas chromatography/mass spectrometry". Journal of Mass Spectrometry 40 (9): 1157–1172. September 2005. doi:10.1002/jms.890. PMID 16041763. Bibcode: 2005JMSp...40.1157T.
- ↑ 3.0 3.1 "#40 2C-T-2". Erowid Online Books : "PIHKAL". http://www.erowid.org/library/books_online/pihkal/pihkal040.shtml.
- ↑ "Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs)". Neuropharmacology 99: 546–553. December 2015. doi:10.1016/j.neuropharm.2015.08.034. PMID 26318099. http://edoc.unibas.ch/56163/1/20170921163006_59c3cceeb8e5d.pdf.
- ↑ "Behavioral and neurochemical pharmacology of six psychoactive substituted phenethylamines: mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function". Psychopharmacology 231 (5): 875–888. March 2014. doi:10.1007/s00213-013-3303-6. PMID 24142203.
- ↑ "Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens". European Neuropsychopharmacology 26 (8): 1327–1337. August 2016. doi:10.1016/j.euroneuro.2016.05.001. PMID 27216487. http://edoc.unibas.ch/53326/1/20170117174852_587e4af45b658.pdf.
- ↑ "Monoamine receptor interaction profiles of 4-thio-substituted phenethylamines (2C-T drugs)". Neuropharmacology. Designer Drugs and Legal Highs 134 (Pt A): 141–148. May 2018. doi:10.1016/j.neuropharm.2017.07.012. PMID 28720478. https://edoc.unibas.ch/57358/1/20170920150712_59c2680084ec5.pdf.
- ↑ Dai, Chongshan; Xiao, Xilong; Sun, Feifei; Zhang, Yuan; Hoyer, Daniel; Shen, Jianzhong; Tang, Shusheng; Velkov, Tony (2019-11-30). "T-2 toxin neurotoxicity: role of oxidative stress and mitochondrial dysfunction". Archives of Toxicology 93 (11): 3041–3056. doi:10.1007/s00204-019-02577-5. ISSN 1432-0738. PMID 31570981. https://pubmed.ncbi.nlm.nih.gov/31570981/.
- ↑ "The neurotoxicity of psychoactive phenethylamines "2C series" in cultured monoaminergic neuronal cell lines" (in en). Forensic Toxicology 38 (2): 394–408. July 2020. doi:10.1007/s11419-020-00527-w. ISSN 1860-8973. https://doi.org/10.1007/s11419-020-00527-w.
- ↑ "2C or not 2C: phenethylamine designer drug review". Journal of Medical Toxicology 9 (2): 172–178. June 2013. doi:10.1007/s13181-013-0295-x. PMID 23494844.
- ↑ "DECRETO 299/2010 - PODER EJECUTIVO NACIONAL (P.E.N.) Estupefacientes - Actualización de la lista y demás sustancias químicas que deberán ser incluidas en los alcances de la ley 23.737 - Sustitución del anexo I del dec. 722/91. Publicado en: BOLETIN OFICIAL 04/03/2010" (in Spanish). 3 February 2010. http://www.mpf.gov.ar/biblioteca/newsletter/n197/DECRETO_299_2010.pdf.
- ↑ "Canada Gazette – Regulations Amending the Food and Drug Regulations (Part J — 2C-phenethylamines)". Government of Canada, Public Works and Government Services Canada, Public Services and Procurement Canada, Integrated Services Branch, Canada. 4 May 2016. http://gazette.gc.ca/rp-pr/p2/2016/2016-05-04/html/sor-dors72-eng.php.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in zh). China Food and Drug Administration. 27 September 2015. http://www.sfda.gov.cn/WS01/CL0056/130753.html.
- ↑ "Förordning (1999:58) om förbud mot vissa hälsofarliga varor". http://www.notisum.se/rnp/sls/fakta/a9990058.HTM.
- ↑ "Läkemedelsverkets författningssamling" (in sv). lakemedelsverket.se. https://lakemedelsverket.se/upload/lvfs/LVFS_2004-3.pdf.
- ↑ "21 U.S. Code § 812 - Schedules of controlled substances" (in en). https://www.law.cornell.edu/uscode/text/21/812.
- ↑ 17.0 17.1 "Poisons Standard October 2015". Federal Register of Legislation. Australian Government, Department of Health, Therapeutic Goods Administration. October 2015. https://www.comlaw.gov.au/Details/F2015L01534.
External links
- 2C-T-2 vault at Erowid
- Sulfurous Samadhi: An Investigation of 2C-T-2 & 2C-T-7
- 2C-T-2 at PsychonautWiki
 |

