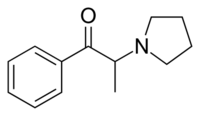
Chemistry:Alpha-Pyrrolidinopropiophenone
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C13H17NO |
| Molar mass | 203.285 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
α-Pyrrolidinopropiophenone (α-PPP), is a stimulant drug. It is similar in structure to the appetite suppressant diethylpropion and has analogous effects in animals. Little is known about this compound, but it has been detected by laboratories in Germany as an ingredient in "ecstasy" tablets seized by law enforcement authorities. This drug has been found to produce stimulant effects in animals and presumably also produces these effects in humans, based on the context in which it has been found.[1][2]
α-PPP is illegal in the UK under the blanket ban on substituted cathinones, and due to its structural similarity to illegal drugs such as methcathinone and pyrovalerone it might be considered a controlled substance analogue in some countries such as the US, Australia and New Zealand. Analogues of α-PPP such as pyrovalerone and MDPV have been more widely used and are presumed to be more potent and addictive than α-PPP itself. Structure-activity relationships of these drugs suggest that a variety of ring-substituted analogues are likely to be potential drugs of abuse,[3] and stimulant activity has been found for analogues with between 3 and 6 carbon atoms in the alkyl chain.[4][5] It has been shown to have neurotoxic effects in animals.[6]
See also
- 4'-Methyl-α-pyrrolidinopropiophenone (MePPP)
- 4'-Methoxy-α-pyrrolidinopropiophenone (MOPPP)
- 3,4-Methylenedioxy-α-pyrrolidinopropiophenone (MDPPP)
- 3',4'-Methylenedioxy-α-pyrrolidinobutiophenone (MDPBP)
- Alpha-Pyrrolidinopentiophenone (α-PVP)
- α-Pyrrolidinopentiothiophenone (α-PVT)
- α-Pyrrolidinohexiophenone (α-PHP)
References
- ↑ "Metabolism of designer drugs of abuse". Current Drug Metabolism 6 (3): 259–74. June 2005. doi:10.2174/1389200054021825. PMID 15975043.
- ↑ "Metabolism of the new designer drug alpha-pyrrolidinopropiophenone (PPP) and the toxicological detection of PPP and 4'-methyl-alpha-pyrrolidinopropiophenone (MPPP) studied in rat urine using gas chromatography-mass spectrometry". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 796 (2): 253–66. November 2003. doi:10.1016/j.jchromb.2003.07.008. PMID 14581066.
- ↑ "Relative reinforcing effects of second-generation synthetic cathinones: Acquisition of self-administration and fixed ratio dose-response curves in rats". Neuropharmacology 134 (Pt A): 28–35. May 2018. doi:10.1016/j.neuropharm.2017.08.018. PMID 28811192.
- ↑ "Chemistry, pharmacology, toxicology, and hepatic metabolism of designer drugs of the amphetamine (ecstasy), piperazine, and pyrrolidinophenone types: a synopsis". Therapeutic Drug Monitoring 26 (2): 127–31. April 2004. doi:10.1097/00007691-200404000-00007. PMID 15228152.
- ↑ "1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors". Journal of Medicinal Chemistry 49 (4): 1420–32. February 2006. doi:10.1021/jm050797a. PMID 16480278.
- ↑ "Effects of the second-generation "bath salt" cathinone alpha-pyrrolidinopropiophenone (α-PPP) on behavior and monoamine neurochemistry in male mice". Psychopharmacology 236 (3): 1107–1117. March 2019. doi:10.1007/s00213-018-5044-z. PMID 30276421.
 |

