Chemistry:2C-O-4
| |
| |
| Names | |
|---|---|
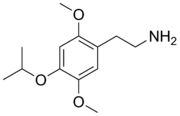
| Preferred IUPAC name
2-{2,5-Dimethoxy-4-[(propan-2-yl)oxy]phenyl}ethan-1-amine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C13H21NO3 | |
| Molar mass | 239.31 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2C-O-4 (4-isopropoxy-2,5-dimethoxyphenethylamine) is a phenethylamine of the 2C family. It is also a positional isomer of isoproscaline and was probably first synthesized by Alexander Shulgin. It produces hallucinogenic, psychedelic, and entheogenic effects.[1] Because of the low potency of 2C-O-4, and the inactivity of 2C-O, Shulgin felt that the 2C-O series would not be an exciting area for research, and did not pursue any further analogues.[1]
Chemistry
2C-O-4 is in a class of compounds commonly known as phenethylamines, and the systematic chemical name is 2-(4-isopropoxy-2,5-dimethoxyphenyl)ethanamine.
Effects
Little is known about the psychopharmacological effects of 2C-O-4. Based on the one report available in his book PiHKAL, Shulgin lists the dosage of 2C-O-4 as being >60 mg.[1]
Pharmacology
The mechanism that produces the hallucinogenic and entheogenic effects of 2C-O-4 is unknown.
Dangers
The toxicity of 2C-O-4 is not known.
Legality
Canada
As of October 31, 2016, 2C-O-4 is a controlled substance (Schedule III) in Canada.[2]
United States
2C-O-4 is unscheduled and unregulated in the United States ; however, because of its close similarity in structure and effects to mescaline and 2C-T-7, possession and sale of 2C-O-4 may be subject to prosecution under the Federal Analog Act.
See also
- 2C-O
- 2C-T-4
References
- ↑ 1.0 1.1 1.2 Shulgin, Alexander; Shulgin, Ann (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628. http://www.erowid.org/library/books_online/pihkal/pihkal.shtml. 2C-O-4 Entry in PiHKAL
- ↑ "Canada Gazette – Regulations Amending the Food and Drug Regulations (Part J — 2C-phenethylamines)". 4 May 2016. http://gazette.gc.ca/rp-pr/p2/2016/2016-05-04/html/sor-dors72-eng.php.
 |

