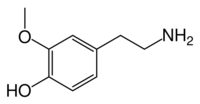
Chemistry:3-Methoxytyramine
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-(2-Aminoethyl)-2-methoxyphenol | |
| Other names
3-O-Methyldopamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| MeSH | 3-methoxytyramine |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H13NO2 | |
| Molar mass | 167.21 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3-Methoxytyramine (3-MT), also known as 3-methoxy-4-hydroxyphenethylamine, is a human trace amine that occurs as a metabolite of the neurotransmitter dopamine.[1] It is formed by the introduction of a methyl group to dopamine by the enzyme catechol-O-methyl transferase (COMT). 3-MT can be further metabolized by the enzyme monoamine oxidase (MAO) to form homovanillic acid (HVA), which is then typically excreted in the urine.
Originally thought to be physiologically inactive, 3-MT has recently been shown to act as an agonist of human TAAR1.[1][2]
Occurrence
3-Methoxytyramine occurs naturally in the prickly pear cactus (genus Opuntia),[3] and is in general widespread throughout the Cactaceae.[4] It has also been found in crown gall tumors on Nicotiana sp.[5]
In humans, 3-methoxytyramine is a trace amine that occurs as a metabolite of dopamine.[1] {{Annotated image 4 | caption = {{{caption|In humans, catecholamines and phenethylaminergic trace amines are derived from the amino acid {{nowrap|L-phenylalanine}}.}}} | header_background = #F0F8FF | header = Biosynthetic pathways for catecholamines and trace amines in the human brain<ref name="Trace amine template 1">Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacol. Ther. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.</ref>[6][7] | alt = Graphic of catecholamine and trace amine biosynthesis | image = Catecholamine and trace amine biosynthesis.png | image-width = 580 | image-left = 5 | image-top = 0 | align = left | width = 590 | height = 585 | annot-font-size = 14 | annot-text-align = center | annotations =
{{annotation|50|565|{{if pagename|Adrenaline=Adrenaline|Epinephrine=Epinephrine|Catecholamine=Epinephrine|other=Epinephrine}}}}
{{annotation|245|60|{{if pagename|Phenethylamine=Phenethylamine|Trace amine=Phenethylamine|Neurobiological effects of physical exercise={{highlight|Phenethylamine}}|other=Phenethylamine}}}}
{{annotation|245|565|{{if pagename|Norepinephrine=Norepinephrine|Adrenaline=Noradrenaline|Catecholamine=Norepinephrine|other=Norepinephrine}}}}
{{annotation|440|295|p-Octopamine}}}}
pathway
CYP2D6
pathway
See also
References
- ↑ 1.0 1.1 1.2 "The emerging roles of human trace amines and human trace amine-associated receptors (hTAARs) in central nervous system". Biomed. Pharmacother. 83: 439–449. October 2016. doi:10.1016/j.biopha.2016.07.002. PMID 27424325.
- ↑ "The dopamine metabolite 3-methoxytyramine is a neuromodulator". PLOS ONE 5 (10): e13452. 2010. doi:10.1371/journal.pone.0013452. PMID 20976142. Bibcode: 2010PLoSO...513452S.
- ↑ Neuwinger, Hans Dieter (1996). "Cactaceae". African ethnobotany: poisons and drugs: chemistry, pharmacology, toxicology. CRC Press. pp. 271. ISBN 978-3-8261-0077-2. https://books.google.com/books?id=_j8ueEmakD0C&pg=PA271. Retrieved on June 12, 2009 through Google Book Search.
- ↑ Smith T. A. (1977). "Phenethylamine and related compounds in plants". Phytochemistry 16 (1): 9–18. doi:10.1016/0031-9422(77)83004-5. Bibcode: 1977PChem..16....9S.
- ↑ Mitchell S. D.; Firmin J. L.; Gray D. O. (1984). "Enhanced 3-methoxytyramine levels in crown gall tumours and other undifferentiated plant tissues". Biochem. J. 221 (3): 891–5. doi:10.1042/bj2210891. PMID 6477503.
- ↑ "A renaissance in trace amines inspired by a novel GPCR family". Trends Pharmacol. Sci. 26 (5): 274–281. May 2005. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- ↑ "The endogenous substrates of brain CYP2D". Eur. J. Pharmacol. 724: 211–218. February 2014. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
{{Navbox
| name = Neurotransmitter metabolism intermediates | title = Neurotransmitter metabolic intermediates | state = autocollapse| | listclass = hlist
| group1 = catecholamines | list1 = {{Navbox|child
| group1 = Anabolism
(tyrosine→epinephrine) | list1 =
- Tyrosine → Levodopa → [[Chemistry:Dop[[Chemistry:Dopamine → Norepinephrine|Norepinephrine]] → Epinephrine
| group2 = Catabolism/
metabolites
| list2 =
| dopamine: | |
|---|---|
| norepinephrine: | |
| epinephrine: |
}}
| group3 = tryptophan→serotonin| list3 =
| anabolism: | |
|---|---|
| catabolism: |


