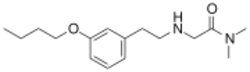
Chemistry:Evenamide
 | |
| Clinical data | |
|---|---|
| Other names | NW-3509; NW-3509A |
| Routes of administration | oral |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C16H26N2O2 |
| Molar mass | 278.396 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Evenamide (INN) (developmental code names NW-3509, NW-3509A) is a selective voltage-gated sodium channel blocker, including (and not limited to) subtypes Nav1.3, Nav1.7, and Nav1.8, which is described as an antipsychotic and is under development by Newron Pharmaceuticals as an add-on therapy for the treatment of schizophrenia.[1][2][3][4] The drug has shown efficacy in animal models of psychosis, mania, depression, and aggression.[3] It has completed phase I clinical trials, and phase II clinical trials will be commenced in the third quarter of 2015.[5]
In a randomized study with treatment-resistant schizophrenia patients, evenamide was added to the treatment regimen, with the psychological assessors being blinded to whether evenamide was taken. 70% of the participants reported a significant lowering of their impairments; and in 25%, schizophrenia went in full remission. A full double-blind phase III study with treatment-resistant schizophrenia patients is in preparation as of January 2023.[6]
See also
References
- ↑ "Drug Development in Schizophrenia: Summary and Table". Pharmaceutical Medicine 28 (5): 265–271. 2014. doi:10.1007/s40290-014-0070-6. ISSN 1178-2595.
- ↑ Progress in Medicinal Chemistry. Elsevier. 6 October 2010. pp. 81–. ISBN 978-0-12-381293-3. https://books.google.com/books?id=dlujSyBq03kC&pg=PA81.
- ↑ 3.0 3.1 Gupta, Satya Prakash (21 June 2011). Ion Channels and Their Inhibitors. Springer Science & Business Media. pp. 102–. ISBN 978-3-642-19922-6. https://books.google.com/books?id=pdnYp8KqJw4C&pg=PA102.
- ↑ "Advances in Design and Development of Sodium Channel Blockers". Ion Channels and Their Inhibitors. 2011. pp. 79–115. doi:10.1007/978-3-642-19922-6_4. ISBN 978-3-642-19921-9.
- ↑ "Newron raised new funds to continue the development of Neurological therapies". Labiotech. 5 February 2015. http://labiotech.eu/newron-raised-new-funds-to-continue-the-development-of-neurological-therapies/.
- ↑ "Newron reports exceptional one-year results of study 014/15 with evenamide in treatment-resistant schizophrenia (TRS)". Newron Pharmaceuticals. 2024-01-04. https://www.newron.com/news-and-media/regulatory-news/newron-reports-exceptional-one-year-results-study-01415-evenamide.
External links
 |

