Chemistry:Pandinotoxin
| Pandinotoxin | |
|---|---|
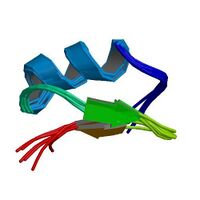 Schematic diagram of the three-dimensional of Pandinotoxin | |
| Identifiers | |
| Organism | |
| Symbol | N/A |
Pandinotoxins are toxins from the venom of the emperor scorpion Pandinus imperator. They are selective blockers of voltage-gated potassium channels [1]
Sources
The source for the pandinotoxins is the venom of the scorpion Pandinus imperator.[1]
Chemistry
Family
The toxins of the family are designated pandinotoxin (PiTX)-Kα, PiTX-Kβ, and PiTX-Kγ [2] They are members of the α-KTx family of scorpion toxins.[1]
Structure and homology
| The sequences of Pandinotoxins |
|---|
|
CTX
PiTXK- α
PiTXK- β
PiTX-K γ
Figure 1: Sequence of Pandinotoxins. Adapted from Solution Structure for Pandinus Toxin K-R (PiTX-KR) [1] |
Pandinotoxin Kα and -β
The amino acid sequences of PiTX-K α and PiTX-K β are identical, except for the seventh amino acid: a proline in PiTX-Kα and a glutamic acid in PiTX-Kβ [2](see Fig.1).
PiTX-Kα and PiTX-Kβ are 35-residue peptides, which are found to have an α-helix from residues 10 to 21 and two β-sheets (β 1 is from residues 26-28, β 2 is from residues 33-35). One face of the α-helix is anchored to the β-sheet by three disulfide bonds which are conserved in all members of the charybdotoxin family (R-K toxins).[1] PiTX-K α and PiTX-K β have only two β-sheets whereas other members of the family have three additional amino acid residues at the N-terminal portion, which forms a third β-sheet.[1]
Pandinotoxin Kγ
Pandinotoxin Kγ has not yet been investigated.
Target
Pandinotoxins are the most potent inhibitors of the rapidly inactivating A-type voltage-gated potassium channels.[3] They also block the delayed rectifier, slowly inactivating channels of the subfamily A member 2 (Kv1.2/KCNA2) [1] and they can reversibly block the shaker B potassium-channels (Kv1.1 sub-family).[4]
Mode of action
The residue K27, a lysine at place 27 of the protein sequence, interacts with the voltage sensitivity blocking activity of CTX channels. It is conserved among PiTX-K α and PiTX-K β. This amino acid is located nearby the selectivity filter of the pore [5] and it is responsible for the interaction with A-type channels by being inserted in the pore of the ion channels.[1] The structural differences in the backbone and side chain between PiTX-Kα and CTX result in a higher affinity for A-type channels for PiTX-Kα.[1] The affinity for the Shaker B K+ channel is significantly smaller for PiTX-Kβ in comparison with PiTX-Kα owing to the changes in the seventh residue.[4]
Therapeutic use
Intraplantarly injection of PiTX-Kα before or after the administration of diclofenac produces a significant reduction in spontaneous flinching, mechanical allodynia and thermal hyperalgesia in a rat model for bone cancer. Downregulation of PiTX-Kα almost completely eliminates diclofenac-induced anti-nociception.[6]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "Solution Structure for Pandinus Toxin K-R (PiTX-KR), a Selective Blocker of A-Type Potassium Channels". Biochemistry 36 (10): 2763–71. 1997. doi:10.1021/bI9628432. PMID 9062103.
- ↑ 2.0 2.1 Rogowski RS; Collins JH; O’Neill TJ; Gustafson TA; Werkman TR; Rogawski MA; Tenenholz TC; Weber DJ et al. (1996). "Three new toxins from the scorpion Pandinus imperator selectively block certain voltage-gated K+ channels". Mol Pharmacol 50 (5): 1167–77. PMID 8913348.
- ↑ "Structural and Functional Differences of Two Toxins From the Scorpion Pandinus Imperator". Proteins 38 (4): 441–9. 2000. doi:10.1002/(sici)1097-0134(20000301)38:4<441::aid-prot9>3.0.co;2-l. PMID 10707030.
- ↑ 4.0 4.1 "Two novel toxins from the venom of the scorpion Pandinus imperator show that the N-terminal amino acid sequence is important for their affinities towards Shaker B K+ channels". J Membr Biol 152 (1): 49–56. 1996. doi:10.1007/s002329900084. PMID 8660410.
- ↑ H. Darbon, E. Blanc; J.M. Sabatier (1999). "Three-dimensional structure of scorpion toxins: Towards a new model of interaction with potassium channels". Perspectives in Drug Discovery and Design 15/16: 41–60. doi:10.1023/A:1017070801207.
- ↑ -Zheng Duan; Qian Xu; Xiao-Meng Zhang; Zhi-Qi Zhao; Yan-Ai Mei; Yu-Qiu Zhang (2012). "Targeting A-type K+ channels in primary sensory neurons for bone cancer pain in a rat mode". Pain 153 (3): 562–574. doi:10.1016/j.pain.2011.11.020. PMID 22188869.
 |
