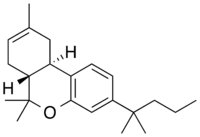
Chemistry:JWH-133
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C22H32O |
| Molar mass | 312.497 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
JWH-133 (Dimethylbutyl-deoxy-Delta-8-THC) is a potent selective CB2 receptor agonist with a Ki of 3.4nM and selectivity of around 200x for CB2 over CB1 receptors. It was discovered by and named after, John W. Huffman.
JWH-133 has been confused with other analogs of Delta-8-THC in peer-reviewed literature. It has been confused with Dimethylpentyl-Delta-8-THC as well as Dimethylbutyl-Delta-8-THC,[1] including confusing the chemical name with Dimethylbutyl-Delta-8-THC itself. It has been confused with the Delta-9 isomer[2]
The 3-(1',1'-Dimethylbutyl)-1-deoxy-delta-8-THC is a selective CB2 agonist, binding 677nM at Cb1 and 3.4nM at CB2[3] while 3-(1',1'-Dimethylbutyl)-delta-8-THC itself binds 65nM at CB1.[4] Structurally the only difference between JWH-133 and dimethylbutyl-D8-THC is that JWH-133 lacks the hydroxy group seen on dimethylbutyl-D8-THCs phenol structure (the C1 position of the A ring), turning this group into a phenyl (JWH-133) instead of phenol.[4][3] It's generally accepted that removing the hydroxy group from the phenol structure of any classical cannabinoid benzoypran (such as THC) results in dramatically less CB1 activity and heightened CB2 activity.[citation needed]
JWH-133, alongside WIN 55,212-2 and HU-210, is responsible for preventing the inflammation caused by Amyloid beta proteins involved in Alzheimer's disease, in addition to preventing cognitive impairment and loss of neuronal markers.[citation needed] This anti-inflammatory action is induced through agonist action at the CB2 receptor, which prevents microglial activation that elicits the inflammation. Additionally, cannabinoids at this receptor completely abolish neurotoxicity related to microglia activation in rat models.[citation needed]
It may be linked with anti-cancer properties, according to pre-trial data from a 2010 study in Madrid.[5]
Legal Status
JWH-133 is not specifically listed in the United States controlled substance act but may be considered an analog of THC (under the Federal Analogue Act) if sold for human consumption.[6]
References
- ↑ "The Structure-Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation". Perspectives in Medicinal Chemistry 8: 17–39. 28 June 2016. doi:10.4137/PMC.S32171. PMID 27398024.
- ↑ "(6AR,10AR)-3-(1,1-Dimethylbutyl)-6A,7,10,10A-tetrahydro-6,6,9-trimethyl-6H-dibenzo[B,D]pyran". https://pubchem.ncbi.nlm.nih.gov/compound/jwh-133.
- ↑ 3.0 3.1 "3-(1',1'-Dimethylbutyl)-1-deoxy-delta8-THC and related compounds: synthesis of selective ligands for the CB2 receptor". Bioorganic & Medicinal Chemistry 7 (12): 2905–2914. December 1999. doi:10.1016/S0968-0896(99)00219-9. PMID 10658595.
- ↑ 4.0 4.1 "Structure-activity relationships for 1',1'-dimethylalkyl-Delta8-tetrahydrocannabinols". Bioorganic & Medicinal Chemistry 11 (7): 1397–1410. April 2003. doi:10.1016/s0968-0896(02)00649-1. PMID 12628666.
- ↑ "Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition". Molecular Cancer 9 (1): 196. July 2010. doi:10.1186/1476-4598-9-196. PMID 20649976.
- ↑ "Federal Register :: Request Access". https://www.ecfr.gov/current/title-21/chapter-II/part-1308.
Further reading
- "Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation". The Journal of Neuroscience 25 (8): 1904–1913. February 2005. doi:10.1523/JNEUROSCI.4540-04.2005. PMID 15728830.
 |

