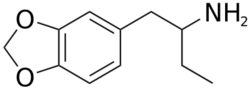
Chemistry:1,3-Benzodioxolylbutanamine
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| Chemical and physical data | |
| Formula | C11H15NO2 |
| Molar mass | 193.246 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 159 to 161 °C (318 to 322 °F) |
| |
| |
| (verify) | |
1,3-Benzodioxolylbutanamine (also known as 3,4-methylenedioxybutanphenamine, MDB, BDB, J, and 3,4-methylenedioxy-α-ethylphenethylamine) is an entactogenic drug of the phenethylamine chemical class.[1][2] It is the α-ethyl analog of MDPEA and MDA and the methylenedioxy analogue of α-ethylphenethylamine.
BDB was first synthesized by Alexander Shulgin. In his book PiHKAL, the dosage range is listed as 150–230 mg and the duration is listed as 4–8 hours.[3] BDB produces entactogenic, MDMA-like effects. Although pleasant and euphoric, BDB is also fairly sedating and some users feel that the lack of stimulant effect makes it less enjoyable than other similar drugs. Additional side effects associated with BDB include nystagmus and dizziness. Very little data exists about the pharmacological properties, metabolism, and toxicity of BDB.
Animal studies and anecdotal reports show that BDB is a slightly more potent serotonin releasing agent than its methylated sister compound methylbenzodioxylbutanamine (MBDB; "Eden", "Methyl-J").[4] However, it is more commonly known as a metabolite of the N-alkylated analogues MBDB and ethylbenzodioxylbutanamine (EBDB; "Ethyl-J") which have appeared in methylenedioxymethamphetamine (MDMA; "Ecstasy", "Adam", "Empathy", "Molly", "E", "X", "XTC") tablets.[5][6] Although BDB itself has not been reported as being sold as "Ecstasy", urine analysis of "Ecstasy" users suggest that this drug may have appeared as a street drug, although it is unclear whether the positive urine test for BDB resulted from consumption of BDB itself or merely as a metabolite of MBDB.[7]
Legal status
1,3-Benzodioxolylbutanamine is illegal in Germany (Anlage I)
See also
- 1-(4-Methylphenyl)-2-aminobutane
References
- ↑ Shulgin, Alexander; Shulgin, Ann (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628. http://www.erowid.org/library/books_online/pihkal/pihkal.shtml.
- ↑ "A behavioral comparison of Nexus, cathinone, BDB, and MDA". Pharmacology, Biochemistry, and Behavior 51 (2–3): 473–5. 1995. doi:10.1016/0091-3057(95)00013-m. PMID 7667371.
- ↑ J entry in PiHKAL
- ↑ "Structure-activity relationships of BDB and its monomethyl and dimethyl derivatives". Pharmacology, Biochemistry, and Behavior 51 (2–3): 477–9. 1995. doi:10.1016/0091-3057(95)00012-l. PMID 7667372.
- ↑ "Excretion of MBDB and BDB in urine, saliva, and sweat following single oral administration". Journal of Analytical Toxicology 21 (7): 570–5. 1997. doi:10.1093/jat/21.7.570. PMID 9399128.
- ↑ "Ion trap mass spectrometry for the characterization of N-methyl-1- (3,4-methylene-dioxyphenyl)-2-butanamine and N-ethyl-3,4- methylenedioxyamphetamine, two widely distributed street drugs". Rapid Communications in Mass Spectrometry 12 (12): 779–82. 1998. doi:10.1002/(SICI)1097-0231(19980630)12:12<779::AID-RCM233>3.0.CO;2-Q. PMID 9650303.
- ↑ "Identification of N-methyl-1-(3,4-methylenedioxyphenyl)-2-butanamine (MBDB) in urine from drug users". Journal of Analytical Toxicology 20 (6): 512–6. October 1996. doi:10.1093/jat/20.6.512. PMID 8889691.
 |

