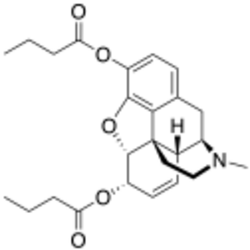
Chemistry:Dibutyrylmorphine
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, intravenous, intranasal, sublingual |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C25H31NO5 |
| Molar mass | 425.525 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Dibutyrylmorphine (also known as dibutanoylmorphine) is the 3,6-dibutyryl ester of morphine, first synthesized by the CR Alders Wright organization in the United Kingdom in 1875.[1]
In animal studies its potency as an analgesic is higher compared to morphine, but lower than that of heroin.[2][3]
Its structure is similar to that of other morphine esters such as heroin and nicomorphine. In many countries it is controlled as an ester of a controlled substance.
Esters of morphine were first produced by boiling morphine in acids or acid anhydrides including acetic, formic, propanoic, benzoic, butyric, and others, forming numerous mono-, di-, and tetraesters.[1] Some of these were later researched further by others and some were eventually marketed.[4] They included heroin, the first designer drugs which were produced in the late 1920s to replace heroin when it was outlawed by the League of Nations, medicinal drugs such as nicomorphine and others. Some of the corresponding esters of codeine, dihydrocodeine, dihydromorphine, isocodeine were also developed, such as the cough suppressant nicocodeine.[5] The 3,6-diesters of morphine are drugs with more rapid and complete central nervous system penetration due to increased lipid solubility and other structural considerations.
References
- ↑ 1.0 1.1 National Research Council US (1941). Report of Committee on Drug Addiction, 1929-1941 and Collected Reprints, 1930-1941. National Academies. https://books.google.com/books?id=n0YrAAAAYAAJ.
- ↑ "Evaluation of 3,6-dibutanoylmorphine as an analgesic in vivo: comparison with morphine and 3,6-diacetylmorphine". Life Sciences 34 (17): 1659–67. April 1984. doi:10.1016/0024-3205(84)90637-4. PMID 6727542.
- ↑ "Relative cataleptic potency of narcotic analgesics, including 3,6-dibutanoylmorphine and 6-monoacetylmorphine". Progress in Neuro-Psychopharmacology & Biological Psychiatry 8 (4–6): 747–50. 1984. doi:10.1016/0278-5846(84)90051-4. PMID 6543399.
- ↑ "Esters of Morphine Opioids". http://www.eopiates.com/opioids/classification/esters-of-morphine-opioids.html.
- ↑ Esters of Morphine, United Nations Office of Drug and Crime
 |

