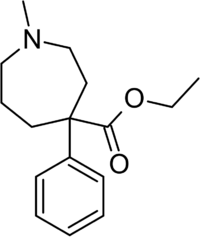
Chemistry:Ethoheptazine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Equagesic |
| Other names | Zactane |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C16H23NO2 |
| Molar mass | 261.365 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ethoheptazine[1] (trade name Zactane) is an opioid analgesic from the phenazepane family. It was invented in the 1950s[2] and is a ring expanded analogue of pethidine.[3]
Ethoheptazine produces similar effects to other opioids, including analgesia, sedation, dizziness and nausea.[4] It was sold by itself as Zactane, and is still available as a combination product with acetylsalicylic acid and meprobamate as Equagesic, which is used for the treatment of conditions where both pain and anxiety are present.[5] It was also investigated for use as an antitussive.[6]
It is no longer prescribed, as it is no longer FDA approved, and not available for United States' Pharmacy Processing. Revocation of FDA Approved Medications Status stems from a combination of efficacy vs. toxicity, and the more-varied and historically safer Benzodiazepines Class. Only reversal of the FDA's decision, allows removing the drug from the CSD. Ethoheptazine is not listed as a controlled substance under the Controlled Substances Act, 1970 in the United States.[7] The controlled status (Schedule IV) of Equagesic was due to the meprobamate content.[8][7] Regulation elsewhere varies.
References
- ↑ "Procedure for the preparation of a new derivative of pirazolidine-hexametilenimina with therapeutic properties." ES patent 310184
- ↑ "Analgesic effectiveness of orally administered ethoheptazine in man". The American Journal of the Medical Sciences 234 (4): 413–9. October 1957. doi:10.1097/00000441-195710000-00004. PMID 13469802.
- ↑ "Synthesis and Properties of the Analgesic DL-α-1,3-dimethyl-4-phenyl-4-propionoxyazacycloheptane (Proheptazine).". Journal of Medicinal Chemistry 7: 57–60. January 1964. doi:10.1021/jm00331a013. PMID 14186026.
- ↑ "[Current pharmaco-therapeutic possiblities in the treatment of pain. Experiments with ethoeptazine]" (in it). Minerva Medica 53: 637–42. March 1962. PMID 13879557.
- ↑ "Treatment of musculoskeletal pain and associated anxiety with an ethoheptazine-aspirin-meprobamate combination (equagesic): a controlled study". Current Therapeutic Research, Clinical and Experimental 16 (9): 928–36. September 1974. PMID 4214668.
- ↑ "Investigation of narcotics and antitussives using drug discrimination techniques". The Journal of Pharmacology and Experimental Therapeutics 211 (2): 401–8. November 1979. PMID 41087.
- ↑ 7.0 7.1 "Conversion Factors for Controlled Substances". Diversion Control Division. Drug Enforcement Agency, U.S. Department of Justice. http://www.deadiversion.usdoj.gov/quotas/conv_factor/index.html.
- ↑ PDR 1978, pp 1618
 |

