Chemistry:Dimethylthiambutene
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
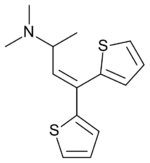
| Formula | C14H17NS2 |
| Molar mass | 263.42 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 169 to 170 °C (336 to 338 °F) |
| |
| |
| (verify) | |
Dimethylthiambutene (N,N-Dimethyl-1-methyl-3,3-di-2-thienylallylamine, DMTB, trade names Ohton, Aminobutene, Dimethibutin, Kobaton, Takaton, Dimethibutin) is an opioid analgesic drug, most often used in veterinary medicine in Japan and to a lesser extent in other countries in the region and around the world. It is the most prominent and widely used of the thiambutenes, a series of open-chain opioids structurally related to methadone which are also called the thienyl derivative opioids which also includes diethylthiambutene and ethylmethylthiambutene, as well as the non-opioid cough suppressant tipepidine.
Dimethylthiambutene was developed in the United Kingdom in the late 1940s and introduced to the market by Burroughs-Wellcome in 1951. Dimethylthiambutene is now under international control under the UN Single Convention on Narcotic Drugs 1961, the laws governing habit-forming substances in virtually all countries and Schedule I of the US Controlled Substances Act of 1970 due to high abuse potential and never being introduced clinically in the United States; other countries regulate it much as morphine or diamorphine. Its DEA ACSCN is 9619 and it had a zero manufacturing quota in 2013.
Synthesis
The conjugate addition between Ethyl crotonate [623-70-1][10544-63-5] (1) and dimethylamine gives Ethyl 3-(Dimethylamino)Butanoate [85118-28-1] (2). Grignard reaction with 2-Bromothiophene [1003-09-4] (3) gives (4). Dehydration in acid completed the synthesis (5).
See also
References
- ↑ "180. Aminoalkyl tertiary carbinols and derived products. Part II. 3-Amino-1: 1-di-2′-thienyl-alkan-1-ols and-alk-1-enes.". Journal of the Chemical Society (Resumed): 885–890. 1950. doi:10.1039/JR9500000885.
- ↑ Wallace AD, US patent 2561899, issued 1951, assigned to Burroughs Wellcome Co.
 |

