Chemistry:Bisdehydrodoisynolic acid
 | |
| Identifiers | |
|---|---|
| |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C18H20O3 |
| Molar mass | 284.355 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
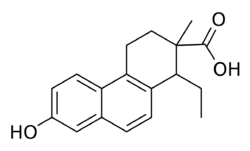
Bisdehydrodoisynolic acid (BDDA), as the (Z)-isomer ((Z)-BDDA), is a synthetic, nonsteroidal estrogen related to doisynolic acid that was never marketed.[1] It is one of the most potent estrogens known,[2][3] although it has more recently been characterized as a selective estrogen receptor modulator (SERM).[3][4] BDDA and other doisynolic acid derivatives display relatively low affinity accompanied by disproportionately high estrogenic potency in vivo,[5] which was eventually determined to be due to transformation into metabolites with greater estrogenic activity.[4] The drug was discovered in 1947 as a degradation product of the reaction of equilenin or dihydroequilenin with potassium hydroxide.[6] It is the seco-analogue of equilenin, while doisynolic acid is the seco-analogue of estrone.[7] These compounds, along with diethylstilbestrol, can be considered to be open-ring analogues of estradiol.[8] The methyl ether of BDDA, doisynoestrol, is also an estrogen, and in contrast to BDDA, has been marketed.[2][9]
See also
References
- ↑ "The Effects of (+)-Z-bisdehydrodoisynolic Acid on Diabetic Phenotype in Female Obese Zucker Rats". Experimental Biology. May 2007. pp. 17–. ISBN 978-0-549-22172-2. https://books.google.com/books?id=L3V0ypHV1H8C&pg=PA17.[yes|permanent dead link|dead link}}]
- ↑ 2.0 2.1 "The Stobbe Condensation with 6-Methoxy-2-propionylnaphthalene. A Synthesis of Bisdehydrodoisynolic Acid1". Journal of the American Chemical Society 72 (2): 925–935. 1950. doi:10.1021/ja01158a075. ISSN 0002-7863.
- ↑ 3.0 3.1 Total Synthesis of Steroids: Organic Chemistry: A Series of Monographs. Elsevier Science. 22 October 2013. pp. 63–. ISBN 978-1-4832-1642-3. https://books.google.com/books?id=Vpb-BAAAQBAJ&pg=PA63.
- ↑ 4.0 4.1 "Derivatives of Z-bisdehydrodoisynolic acid provide a new description of the binding-activity paradox and selective estrogen receptor modulator activity". Endocrinology 147 (8): 3952–3960. August 2006. doi:10.1210/en.2006-0316. PMID 16709609.
- ↑ "Comparative effects of the selective estrogen receptor modulators (-)-, (+)- and (+/-)-Z bisdehydrodoisynolic acids on metabolic and reproductive parameters in male and female rats". Hormone and Metabolic Research 30 (12): 730–736. December 1998. doi:10.1055/s-2007-978968. PMID 9930631.
- ↑ The Hormones V1: Physiology, Chemistry and Applications. Elsevier. 2 December 2012. pp. 364–366. ISBN 978-0-323-14206-9. https://books.google.com/books?id=Thtz7On_lhEC&pg=PA364.
- ↑ Journal of Scientific & Industrial Research. Council of Scientific & Industrial Research. 1984. p. 213. https://books.google.com/books?id=0Z0jAAAAMAAJ.
- ↑ "Ring transformations". The Practice of Medicinal Chemistry. Academic Press. 2 May 2011. pp. 343-362 (344). ISBN 978-0-08-056877-5. https://books.google.com/books?id=Qmt1_DQkCpEC&pg=PA344.
- ↑ "Doisynoestrol". The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 465–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA465.
 |
