Chemistry:Equilin
 | |
| Clinical data | |
|---|---|
| Other names | Δ7-Estrone; 7-Dehydroestrone; Estra-1,3,5(10),7-tetraen-3-ol-17-one |
| Routes of administration | By mouth |
| Drug class | Estrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H20O2 |
| Molar mass | 268.356 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Equilin is a naturally occurring estrogen sex hormone found in horses as well as a medication.[1][2][3] It is one of the estrogens present in the estrogen combination drug preparations known as conjugated estrogens (CEEs; e.g. Premarin) and esterified estrogens (EEs; e.g. Estratab, Menest).[2][3] CEEs is the most commonly used form of estrogen medications in hormone replacement therapy (HRT) for menopausal symptoms in the United States .[3] Estrone sulfate is the major estrogen in CEEs (about 50%) while equilin sulfate is the second major estrogen in the formulation, present as about 25% of the total.[2][3]
Pharmacology
Pharmacodynamics
Equilin is an estrogen, or an agonist of the estrogen receptors (ERs), the ERα and ERβ.[2] In terms of relative binding affinity for the ERs, equilin has about 13% and 49% of that of estradiol for the ERα and ERβ, respectively.[2] Analogously to the reversible transformation of estrone into estradiol by 17β-hydroxysteroid dehydrogenase, equilin can be converted into the more potent estrogen 17β-dihydroequilin in the body.[2][3] This estrogen has about 113% and 108% of the relative binding affinities of estradiol for the ERα and ERβ, respectively.[2][3] Equilin is present in CEEs in the form of equilin sulfate, which itself is inactive and acts as a prodrug of equilin via steroid sulfatase.[2][3]
Similarly to synthetic estrogens like ethinylestradiol, equilin and CEEs have disproportionate effects in certain tissues such as the liver and uterus relative to bioidentical human estrogens like estradiol and estrone.[2] Because of their disproportionate potency in the liver, equilin and CEEs have relatively increased effects on liver protein synthesis compared to estradiol.[2]
A dosage of 0.25 mg/day equilin sulfate is equivalent to 0.625 mg/day CEEs in terms of relief from hot flashes.[2] At a dosage of 0.625 mg/day equilin sulfate, the increases in circulating levels of sex hormone-binding globulin (SHBG), corticosteroid-binding globulin, and angiotensinogen were 1.5 to 8 times those observed with estrone sulfate.[2] Equilin has about 42% of the relative potency of CEEs in the vagina and 80% of the relative potency of CEEs in the uterus, while its more active form, 17β-dihydroequilin, has about 83% of the relative potency of CEEs in the vagina and 200% of the relative potency of CEEs in the uterus.[2]
Pharmacokinetics
Equilin has about 8% of the relative binding affinity of testosterone for SHBG, relative to 12% in the case of estrone.[2] In terms of plasma protein binding, it is bound 26% to SHBG and 13% to albumin.[2] The metabolic clearance rates of equilin and equilin sulfate are 2,640 L/day/m2 and 175 L/day/m2, respectively.[2] In accordance, the biological half-life of equilin sulfate is substantially longer than that of equilin.[2] Equilin is converted into 17β-dihydroequilin in the liver and in other tissues.[2][3] Equilin and 17β-dihydroequilin can also be transformed into equilenin and 17β-dihydroequilenin.[2][3] Equilin is excreted in the form of glucuronide conjugates.[2]
Chemistry
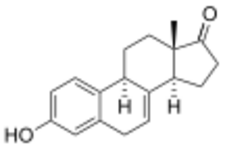
Equilin, also known as δ7-estrone or as 7-dehydroestrone, as well as estra-1,3,5(10),7-tetraen-3-ol-17-one, is a naturally occurring estrane steroid and an analogue of estrone.[2][3] In terms of chemical structure and pharmacology, equilin is to 17β-dihydroequilin (δ7-17β-estradiol) as estrone is to estradiol.[2][3]
References
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. p. 495. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA298.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric 8 (Suppl 1): 3–63. 2005. doi:10.1080/13697130500148875. PMID 16112947. http://hormonebalance.org/images/documents/Kuhl%2005%20%20Pharm%20Estro%20Progest%20Climacteric_1313155660.pdf.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 "Pharmacology of conjugated equine estrogens: efficacy, safety and mechanism of action". J. Steroid Biochem. Mol. Biol. 142: 16–29. July 2014. doi:10.1016/j.jsbmb.2013.10.011. PMID 24176763.
 |
