Chemistry:Triphenyliodoethylene
From HandWiki
Short description: Chemical compound
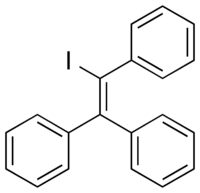 | |
| Clinical data | |
|---|---|
| Other names | TPIE; Iodotriphenylethylene; Phenylstilbene iodide; Triphenylvinyl iodide |
| Drug class | Nonsteroidal estrogen |
| Identifiers | |
| |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C20H15I |
| Molar mass | 382.244 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Triphenyliodoethylene (TPIE), also known as iodotriphenylethylene or as phenylstilbene iodide, as well as triphenylvinyl iodide, is a synthetic nonsteroidal estrogen of the triphenylethylene group that is related to triphenylchloroethylene and triphenylbromoethylene and was never marketed.[1][2][3]
See also
References
- ↑ "Synthetic oestrogens related to triphenylethylene". Proceedings of the XIth International Congress of Pure and Applied Chemistry: Chemistry in relation to medicine and theropeutics [sic], chemistry in relation to fuel, power and transport. Hepworth. 1947. p. 149. https://books.google.com/books?id=bMMfAQAAMAAJ. "In fact the cestrogenic activity of either triphenylbromoethylene or triphenyliodoethylene (J. 3d. Robson, A. Schonberg and H. A. Fahim)(3) (Table 1) compares with that of triphenylchloroethylene. A True Oestrogen and A Pro-oestrogen."
- ↑ British Abstracts. Bureau of Abstracts. 1952. p. 549. https://books.google.com/books?id=D0E_AAAAYAAJ. "Roughly quantitative data are reported for the antagonism between oestrogens (oestradiol, stilboestrol, doisynolic acid, allenolic acid, and triphenyliodoethylene) and progesterone, methyltestosterone, and testosterone propionate given [...]"
- ↑ Egyptian Veterinary Medical Association (1966). Annual Veterinary Congress, Proceedings. L'Institut Francais d'Archéologie Orientale. p. 392. https://books.google.com/books?id=vztXAAAAYAAJ. "But the synthetic oestrogens tested e.g. stilboesterol, triphenyliodoethylene, diosynolic acid and allenolic acid produce constant inhibition of the uterine motility."
 |

