Chemistry:Doisynolic acid
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C18H24O3 |
| Molar mass | 288.387 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
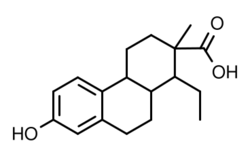
Doisynolic acid is a synthetic, orally active, nonsteroidal estrogen that was never marketed.[1][2][3] The reaction of estradiol or estrone with potassium hydroxide, a strong base, results in doisynolic acid as a degradation product, which retains high estrogenic activity, and this reaction was how the drug was discovered, in the late 1930s.[4][5][6] The drug is a highly active and potent estrogen by the oral or subcutaneous route.[4] The reaction of equilenin or dihydroequilenin with potassium hydroxide was also found to produce bisdehydrodoisynolic acid, whose levorotatory isomer is an estrogen with an "astonishingly" high degree of potency, while the dextrorotatory isomer is inactive.[4] Doisynolic acid was named after Edward Adelbert Doisy, a pioneer in the field of estrogen research and one of the discoverers of estrone.[7]
Doisynolic acid is the parent compound of a group of synthetic, nonsteroidal estrogens with high oral activity.[8] The synthetic, nonsteroidal estrogens methallenestril, fenestrel, and carbestrol were all derived from doisynolic acid and are seco-analogues of the compound.[9] Doisynoestrol, also known as fenocycline, is cis-bisdehydrdoisynolic acid methyl ether, and is another estrogenic derivative.[10]
See also
References
- ↑ Dictionary of Steroids. CRC Press. 23 May 1991. pp. 422–. ISBN 978-0-412-27060-4. https://books.google.com/books?id=AI7EnUyeEtUC&pg=PA422.
- ↑ "Estrogens, Antiestrogens, and Other Estrane Compounds". Antitumor Steroids. Academic Press. 2 December 2012. pp. 11–12. ISBN 978-0-323-13916-8. https://books.google.com/books?id=sxMBCIoDS1MC&pg=PA11.
- ↑ "Mechanims of Action of Estrogens". Antineoplastic and Immunosuppressive Agents. Springer Science & Business Media. 27 November 2013. pp. 106–. ISBN 978-3-642-65806-8. https://books.google.com/books?id=aU_oCAAAQBAJ&pg=PA106.
- ↑ 4.0 4.1 4.2 "The Chemistry and Metabolism of the Estrogens". The Hormones V1: Physiology, Chemistry and Applications. Elsevier. 2 December 2012. pp. 364–366. ISBN 978-0-323-14206-9. https://books.google.com/books?id=Thtz7On_lhEC&pg=PA364.
- ↑ "Chapter 3.4: 18,19-Norprogesterone and 19-Norpreganes". Total Synthesis of Steroids: Organic Chemistry: A Series of Monographs. Elsevier Science. 22 October 2013. pp. 65–. ISBN 978-1-4832-1642-3. https://books.google.com/books?id=Vpb-BAAAQBAJ&pg=PA65.
- ↑ "Estrogen, Progestins, and Androgens". Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. January 2002. pp. 692–. ISBN 978-0-683-30737-5. https://books.google.com/books?id=qLJ6Bs1Qml4C&pg=PA692.
- ↑ "Synthetic oestrogens". British Medical Bulletin 11 (2): 131–134. May 1955. doi:10.1093/oxfordjournals.bmb.a069465. PMID 14378564.
- ↑ "Estrogens". Chemical Endocrinology. Elsevier Science. 2 December 2012. pp. 53–. ISBN 978-0-323-15906-7. https://books.google.com/books?id=CRQ-KO0qsT0C&pg=PA53.
- ↑ Encyclopedia of chemical technology. Wiley. 1980. p. 670,672. ISBN 978-0-471-02065-3. https://books.google.com/books?id=yb9TAAAAMAAJ.
- ↑ The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 465–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA465.
 |

